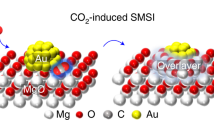
Abstract
Key chemical transformations require metal and redox sites in proximity at interfaces; however, in traditional oxide-supported materials, this requirement is met only at the perimeters of metal nanoparticles. We report that galvanic replacement can produce inverse FeOx/metal nanostructures in which the concentration of oxide species adjoining metal domains is maximal. The synthesis involves reductive deposition of rhodium or platinum and oxidation of Fe2+ from magnetite (Fe3O4). We discovered a parallel dissolution and adsorption of Fe2+ onto the metal, yielding inverse FeOx-coated metal nanoparticles. This nanostructure exhibits the intrinsic activity in selective CO2 reduction that simple metal nanoparticles have only at interfaces with the support. By enabling a simple way to control the surface functionality of metal particles, our approach is not only scalable but also enables a versatile palette for catalyst design.
Similar content being viewed by others
Introduction
Metal particles play a key role in chemical transformations that require activation of H2 or hydrogenation/dehydrogenation of substrates. In many cases, the metal particles provide only one step in the catalytic cycle. For instance, metals have low activity in CO2 reduction because of weak CO2 adsorption, whereas the polar surface of oxides readily adsorbs CO2 but suffers from low activity for H2 activation1,2,3. Thus, metal–oxide interfaces are much more effective because both the redox sites required to activate CO2 and the metals providing active H2 are in proximity. Challenges for maximizing such interfaces are stabilizing small metal particles on oxide supports4,5,6,7 or forcing migration of oxides onto metal particles while avoiding harsh synthesis conditions8,9,10,11,12.
Inverse catalysts—oxides supported on metals—offer an attractive alternative to overcome the constraints of typical supported metal catalysts because reactants can bind to sites in the oxide overlayer, onto the metal domains, or at their interface. Typically, surface science research selects only well-defined inverse catalysts to provide a basic understanding of their adsorption and catalytic properties; however, advancing from this approach into the more complex conditions relevant to technical applications is essential13,14,15,16. In this regard, a major obstacle is encountered because typical surface science approaches for preparing inverse catalysts, such as reduction at high temperature12, deposition in ultrahigh vacuum1,13, and deposition at atomic layers17, are challenging to scale beyond certain models.
We report here a simple galvanic replacement approach for generating inverse FeOx/metal nanostructures. During galvanic replacement, one metal dissolves as a sacrificial template while a different metal ion in solution is reductively deposited onto the template. This process is driven by the differences of reduction potentials of the redox pairs, allowing a single, simple, and low-temperature step for synthesis of nanostructures18,19,20,21,22,23. Following this, research has focused on preparing metals18, metal alloys19,24, oxides21, and metal–oxides25,26 with controllable shapes. In our case, the solid support undergoing oxidation—hyperstoichiometric and sometimes referred to as cation-excess or partially reduced magnetite (Fe3O3.7)27—supplies electron equivalents in the form of Fe2+ enriched at the oxide surface, which reduce Rh3+ or Pt4+, thereby depositing metal nanostructures (Eqs. (1)–(3)).
We discovered that in addition to acting as sacrificial species, Fe2+ dissolves, and adsorbs onto the as-formed metal particles as Fe(II)-oxyhydroxide. The surface property of the metal is thus greatly changed by the FeOx overlayer, endowing the nanostructure with the high density of active sites for CO2 reduction that well-dispersed Rh particles have only at the interface with Fe3O4. This yields activity and selectivity for CO production significantly higher than well-dispersed Rh particles without FeOx overlayers. Our method demonstrates that the surface of metal nanoparticles can be manipulated by the sacrificial species during galvanic replacement, whereas galvanic replacement was previously thought to control only nanostructure morphologies.
Results and discussion
Identification of FeOx overlayer on Rh
We performed the synthesis by simply suspending Fe3O3.7 (“Methods” section and Supplementary Fig. 1 for synthesis) in aqueous RhCl3 solution (Fig. 1a), yielding the as-prepared material (FeOx/Rh/Fe3O4-fresh). High-angle annular dark-field scanning tunneling electron microscopy (HAADF-STEM) imaging of FeOx/Rh/Fe3O4-fresh (Fig. 1b and Supplementary Figs. 2–5) showed that the deposited nanostructures distribute along the whole surface of Fe3O4 with the average size of 6.6 nm. These nanostructures seem to be composed by smaller Rh nanoparticles of around 2 nm. The nanosized structures were further examined by electron energy-loss spectroscopy (EELS) while manipulating the sample to avoid overlapping with the support along z-axis. Maps of Rh L2,3 and Fe L2,3 edges (Fig. 1b and Supplementary Figs. 3–5) show Fe signals in regions of the Rh domains. The line profile indicates that significant amounts of Fe coincide with Rh particles. The Fe spectra from the Rh domains give a lower loss energy (by 0.7 eV) than the signal from Fe3O4 (Supplementary Fig. 3). This indicates that the Fe on Rh nanostructures have a lower average oxidation state (i.e., +2) than that in Fe3O4 (+8/3).
Synthesis of FeOx/Rh/Fe3O4-fresh by galvanic replacement, where powder Fe3O3.7 is contacted with a solution containing Rh3+ (a). Rh and Fe L-edges EELS images of the FeOx/Rh/Fe3O4-fresh and the corresponding line-scan profile showing the FeOx coating on Rh; the scale bar is 5 nm (b); more images can be found in Supplementary Figs. 2–5. Evolution of concentrations of aqueous Fe2+ and Fe3+ when Fe3O3.7 is contacted with the Rh3+ solution (c). Scheme of the reference experiments, where pre-formed Rh nanoparticles were contacted with solutions containing Fe2+ or Fe3+ (d). HAADF-STEM-EELS images of the solids produced after contacting Rh nanoparticles with Fe2+ (e) or Fe3+ (f) in solution showing the selective adsorption of Fe2+ on Rh producing the Fe(II)-oxyhydroxide adlayers on Rh.
Rh K-edge X-ray absorption near edge structure (XANES, Supplementary Fig. 3) showed that the white-line of FeOx/Rh/Fe3O4-fresh is similar to that of Rh foil. Linear combination fitting indicated that 77 mol.% of Rh is metallic (Supplementary Fig. 3 and Supplementary Table 1). This agrees well with the Rh extended X-ray absorption fine-structure (EXAFS) fitting showing that the Rh species have high Rh–Rh coordination (Supplementary Fig. 6 and Supplementary Table 2). The fitting of the spectra required a Rh–O path with a coordination number of 1.9 ± 0.5. Thus, 23 mol.% of Rh remains oxidized, probably because of its interaction with the FeOx species (Supplementary Table 1). The results indicated that inverse FeOx/Rh nanostructures were formed where Rh was reductively deposited while Fe(II)-oxyhydroxide species bind onto the Rh.
Mechanism for the formation of FeOx overlayer
We used the ferrozine method28 to monitor changes of Fe3+ and Fe2+ concentrations during synthesis of FeOx/Rh/Fe3O4-fresh (Fig. 1c). Fe2+ was released immediately after Fe3O3.7 was dispersed in the solution of Rh3+, which is consistent with the acidic Fe3O4 oxidation chemistry (Eq. (4))27 because Fe2+ is much more soluble than Fe3+, and the solution is initially free of aqueous Fe2+ upon first contact the Rh3+ solution. The pH was observed to initially drift upwards from ~4 to 5, which is consistent with consumption of protons during the release (see section of methods for pH changes).
The particle surface will be enriched in Fe2+ during the Fe2+ release into solution29, thereby maintaining a dynamic equilibrium27. In parallel, Rh3+ was reduced and deposited as the nanostructures that adsorb and bind Fe(II)-oxyhydroxide during the progressive Fe2+ accumulation on the Fe3O4 surface (see below). This leads to a gradual reversal of the reaction in Eq. (4), detectable by a pH decrease from ~5 to 2.5 and an increase in Fe3+ in solution, reaching equilibrium after 3 h synthesis time. Note that if Rh3+ and Fe2+ (Rh3+:Fe2+ = 1:3) were mixed at the conditions of the galvanic replacement, neither Rh nor FeOx particles are observed by HAADF-STEM. Thus, Rh nucleation and growth requires the Fe3O4 surface and productions of Fe2+ and Fe3+ in solution follow different mechanisms.
Because formation of metallic Rh is accompanied by increasing detectable aqueous Fe3+ (Eq. (3)) and consumption of Fe2+ (Fig. 1c), we attribute the Fe(II)-oxyhydroxide coating on Rh particles to the dynamic equilibrium of the Fe2+ release process (i.e., the reverse of Eq. (4))27,29. To confirm the selective interaction of Fe2+ with Rh, we contacted pre-formed Rh nanoparticles with solutions containing either Fe2+ or Fe3+ cations and analyzed the recovered particles (Fig. 1d). The syntheses of these reference materials are described in the section of methods. The EELS images showed that Fe2+ species adsorb on the Rh surface to form a core-shell-like nanostructure (Fig. 1e), whereas Fe3+ species precipitate as a segregated phase with only weak association with Rh (Fig. 1f).
Overall, the charge transfer of galvanic replacement consumes Fe2+ supplied by Fe3O4 for Rh3+ reduction yielding Rh particles (Fig. 2a). In parallel, Fe2+ released from the solid (Fig. 2b) adsorbs selectively on Rh (Fig. 2c). In order to verify the generality of our methodology to prepare inverse nanostructures, we also performed the galvanic replacement between the Pt4+ cations and Fe3O3.7. The HAADF-STEM-EELS showed that FeOx species coat the Pt nanoparticles (Supplementary Fig. 7). Hence, during the synthesis of FeOx/metal nanostructures, the Fe2+ is not only a sacrificial species as one expects from the galvanic replacement alone, but a key constituent for tuning the surface of the metal nanoparticles. The method offers many possibilities to tune the properties and structures of the final materials by controlling the rates of the individual processes taking place during the synthesis. Further work to control the metal particle size and FeOx coverage is ongoing.
Comparison of FeOx-covered and bare Rh particles supported on Fe3O4
We compared the inverse catalyst with Fe3O4-supported 1–2 nm Rh particles (Rh/Fe3O4) in CO2 hydrogenation. This reference was prepared by precipitating Rh3+ on Fe3O4 followed by treatment in air and reduction at 200 °C in H2 (Supplementary Fig. 8 for the Rh particle size distribution). To remove possible adsorbates remaining from synthesis and handling, the FeOx/Rh/Fe3O4-fresh material was treated at the same conditions as Rh/Fe3O4, yielding the material denoted as FeOx/Rh/Fe3O4. This material showed the same features of the parent FeOx/Rh/Fe3O4-fresh. That is, the dispersed FeOx species still decorated the metallic Rh particles (Fig. 3a–f and Supplementary Fig. 9). The inverse FeOx/Rh nanostructure was unaltered by the heat treatment, in agreement with the lower surface energy of iron oxide (Fig. 3h and Supplementary Table 3), which tends to wet the Rh surface13.
According to XANES (Fig. 3g) and EXAFS results (Fig. 4a), Rh in both FeOx/Rh/Fe3O4 and Rh/Fe3O4 are mainly metallic with a Rh–Rh distance of 2.68 Å. The Rh–Rh coordination number for FeOx/Rh/Fe3O4 is ~8.9 while for Rh/Fe3O4 it is 6.7 (Supplementary Tables 4 and 5 and Supplementary Figs. 10 and 11). This suggests that the Rh dispersion of FeOx/Rh/Fe3O4 was lower than Rh/Fe3O4 (i.e., 56% and 82%, respectively) (Supplementary Fig. 12). The difference in Rh dispersion was supported by time-of-flight secondary ion mass spectrometry (TOF-SIMS), which showed more abundant Rh2O+ fragments for FeOx/Rh/Fe3O4 than for Rh/Fe3O4 (Supplementary Fig. 13).
Rh K-edge EXAFS spectra (a), isotherms of H2 and CO2 chemisorption (b, c), CO2 conversion rates (d), and CO selectivity for CO2 conversion (e), and the Arrhenius plots for CO2 reduction to CO (f) for FeOx/Rh/Fe3O4 and Rh/Fe3O4. Reaction conditions: 523–623 K, 101 kPa, CO2/H2/He = 7/28/105 mL min−1 (gas hourly space velocity of 7 × 105 mL g−1 h−1). The CO2 and CO rates are normalized to the concentration of surface Rh as derived from EXAFS fitting.
In contrast to the EXAFS of FeOx/Rh/Fe3O4 showing 56% Rh dispersion, H2 chemisorption indicates that only 5.6% of Rh is available to adsorb H2 (Fig. 4b and Supplementary Table 6). The discrepancy between EXAFS and H2 chemisorption is clearly due to the presence of FeOx overlayer. For Rh/Fe3O4, H2 chemisorption suggests a 70% dispersion, which is in good agreement with EXAFS results (i.e., most of the surface Rh atoms in the nanoparticles are available to adsorb H2). Both FeOx/Rh/Fe3O4 and Rh/Fe3O4 have the same adsorption equilibrium constant for H2 chemisorption (~7, Supplementary Table 6). Therefore, metallic Rh atoms are the sites for H2 activation on both materials.
Catalytic improvement by the FeOx overlayer
We targeted CO2 hydrogenation to test the activity of our inverse catalysts. Thus, we measured isotherms for CO2 adsorption (Fig. 4c), which showed that FeOx/Rh/Fe3O4 can adsorb more CO2 than Rh/Fe3O4 and pure Fe3O4 (Supplementary Table 6) (i.e., 7.2, 4.3, and 2.2 μmolCO2 g−1, respectively, at 33 kPa). The adsorption equilibrium constant for FeOx/Rh/Fe3O4 also is higher than for Rh/Fe3O4 (i.e., 100 and 51, respectively) (Fig. 4c and Supplementary Table 6). Thus, the adsorption sites on the inverse FeOx/Rh catalyst have stronger interactions with CO2 than the sites in Rh/Fe3O4. The differences in adsorption capacity and strength have important consequences in the coverages of molecular species during the reaction (Supplementary Table 7 and Supplementary Figs. 14 and 15), and thus the catalytic performance described below.
The inverse FeOx/Rh/Fe3O4 catalyst showed high activity for CO2 reduction per mol of surface Rh (determined from the Rh–Rh coordination number from EXAFS analysis) compared to that of Rh/Fe3O4 (Fig. 4d). We used this normalization to reflect the surface of the catalysts that is potentially active, i.e., Rh with or without interactions with the support (note however, these trends are the same per mass of catalyst and mass of Rh). The selectivity to CO and the corresponding CO production rates are also higher on FeOx/Rh/Fe3O4 than on Rh/Fe3O4 (Fig. 4e, f). This highlights the higher activity of the FeOx-coated particles than simple supported Rh particles. We analyzed the intrinsic activity of the materials not by normalizing rates to the fraction of exposed Rh (as determined from H2 chemisorption) nor to the fraction of Rh covered by oxide species (Supplementary Table 8 and Supplementary Note). Instead, we considered that the uptake of CO2 serves as titration of adsorption sites that can potentially produce CO (see the supporting information for more details). The rates of CO production normalized to the concentration of sites that chemisorb CO2 were, e.g., 1657 and 1222 h−1 on FeOx/Rh/Fe3O4 and Rh/Fe3O4, respectively, at 250 °C. The similarity of these values, and of the activation energies for CO production (Fig. 4f), allows us concluding that the highly active and selective sites in both systems are similar. These sites, in view of the negligible activity of SiO2-supported Rh and pure Fe3O4 (Supplementary Table 8) are undoubtedly identified as Rh–Fe3O4 interfaces30,31,32,33.
We also tested the FeOx/Rh nanoparticles (Fig. 1e) and the parent Rh nanoparticles in CO2 reduction (Supplementary Table 9). The FeOx/Rh nanoparticles were one order of magnitude more active than the bare Rh nanoparticles. The bare Rh nanoparticles produced both CO and methane in equimolar concentrations, while FeOx/Rh nanoparticles selectively yielded CO. These observations further support our claim that the FeOx adlayers increase the activity for CO2 conversion and the selectivity to CO. The FeOx/Rh nanoparticles, however, led to 1–2 orders of magnitude lower rate for CO2 reduction than the inverse FeOx/Rh/Fe3O4 catalyst, which highlights the role of the Fe3O4 support, which maintains the FeOx/Rh nanoparticles separated.
The FeOx/Rh/Fe3O4 inverse catalyst is also more productive than typical supported noble-metal nanoparticles and atomically dispersed Rh (Supplementary Table 10). Thus, leaving some exposed Rh on the surface of FeOx/Rh/Fe3O4 does not lead to low activity because the surface behaves like Rh–Fe3O4 interfaces.
In summary, this galvanic replacement approach to prepare inverse FeOx/metal nanostructures not only yields particularly compelling catalytic reactivity under real conditions but is versatile and easily scalable13,17,34. The ability to control the surface functionality of metal nanoparticles enables a palette for catalyst design via galvanic replacement. The presence of the oxide overlayer makes the metal much more efficient for activating CO2 while maintaining its hydrogenation ability. That is, the whole surface of the metal particle functions as metal/oxide interface with redox sites for adsorbing CO2 near metal domains that dissociate H2 but with limited capacity to produce methane.
Methods
Materials
The chemicals including magnetite (Fe3O4) nanoparticles (50–100 nm), RhCl3 (37% Rh), rhodium (III) nitrate hydrate (Rh(NO3)3·H2O), FeCl2 (≥99.0%), FeCl3 (≥99.0%), urea (99.0–100.5%), polyvinylpyrrolidone (PVP), and ethylene glycol were purchased from Sigma-Aldrich. The deionized water was obtained from a Milli-Q water system.
Synthesis of FeOx/Rh/Fe3O4-fresh, FeOx/Rh/Fe3O4 and FeOx/Pt/Fe3O4-fresh
The FeOx/Rh/Fe3O4-fresh with the pre-set Rh loading of 0.5 wt% was prepared by galvanic replacement between Rh3+ and partially reduced magnetite (Fe3O3.7). In a typical procedure, 9.95 g of Fe3O4 was reacted in 5 vol.% H2/N2 at 400 °C in a tube furnace to produce Fe3O3.7. The Fe3O4 symmetry group remained for Fe3O3.7 after this step (Supplementary Fig. 1). A 10 mL aqueous solution of RhCl3 at a concentration of 5 mgRh mL−1 was mixed with 90 mL deionized water at room temperature. The Fe3O3.7 was then added to the solution and stirred for 7 h. The resulting material was separated, washed with water, and dried at 80 °C overnight. The as-prepared material was calcined in air at 450 °C with a ramping rate of 2 °C/min. The inductively coupled plasma (ICP) analysis showed that the effective Rh loading in the final material was 0.37 wt%. Prior to the catalytic test, the sample was treated at 200 °C in H2. The purpose of heat treatments is to remove the possible surface ligands and surface-oxidized Rh species that remained during the synthesis. A material containing Pt (FeOx/Pt/Fe3O4-fresh) was prepared by the same method with aqueous solution of H2PtCl6 as the precursor and a pre-set Pt loading of 0.5 wt%.
The dynamic changes of the Fe2+ and Fe3+ concentrations in the aqueous fraction during the galvanic replacement synthesis (for FeOx/Rh/Fe3O4-fresh) were analyzed by the ferrozine method28. The suspension was centrifuged to isolate the aqueous fraction during the galvanic replacement (0, 0.1, 0.5, 1, 2, 3, 4, 5, 6, and 7 h). The pH values for the aqueous solutions increased slightly at first (from 4.0 to 5.0), and then decreased to ~2.5. The resulting aqueous solutions were diluted in a 10−2 M HCl solution and used for the analysis. The Fe2+ can react with ferrozine to form a stable magenta complex which gives a maximum absorbance at 562 nm on an ultraviolet–vis spectrophotometer. The Fe3+ fraction can be detected by reducing with hydroxylamine hydrochloride solution, stabilized in a buffer, and followed by complexing with ferrozine.
Synthesis of Rh/Fe3O4
The Rh/Fe3O4 with a Rh loading of 0.5 wt% was prepared by a urea hydrolysis assisted deposition method. In a typical procedure, 9.95 g of Fe3O4 were dispersed in 100 mL deionized water. Then, a 10 mL aqueous solution of RhCl3 at a concentration of 5 mgRh mL−1 was added into the suspension and rigorously stirred for 12 h at room temperature. An excess of urea (urea/[Rh] molar ratio = 60) was added to the suspension for deposition of Rh3+. The Rh3+ can be deposited homogeneously and slowly with the help of urea hydrolysis in a hydrothermal condition (90 °C) for 6 h. The resulting material was separated, washed with water, and dried at 80 °C overnight. The as-prepared material was treated in air at 450 °C with a ramping rate of 2 °C min−1. The ICP results suggested that the Rh loading was 0.37 wt%. Prior to the catalytic test, the sample was treated at 200 °C in H2. A reference Rh/SiO2 with the Rh loading of 0.5 wt% was also prepared by the urea hydrolysis deposition method, followed by the same treatments before catalytic test.
Synthesis of Rh nanoparticles (PVP method)
In a typical procedure, Rh nanoparticles were synthesized following a polyol-based method. Rh nitrate (Rh amount 100 mg) was dispersed in 60 mL of ethylene glycol in the presence of a stabilizer (PVP) and heated under reflux for 6 h. The Rh nanoparticles then were washed with acetone and water eight times before used for model synthesis experiments.
Mixing of Rh3+ and Fe2+ cations in solution in the absence of solid
In a typical procedure, 0.25 mL RhCl3 aqueous solution (5 mg[Rh] mL−1), 1 mL FeCl2 aqueous solution (2 mg[Fe] mL−1), and 4 mL deionized water were mixed at room temperature and stirred for 7 h. This procedures were perfomed in a N2 glove box.
Reaction of Rh0 nanoparticle and Fe2+ cations
After washing three times with deionized water, 1.25 mg Rh0 nanoparticles were dispersed in 4 mL deionized water and mixed with 1 mL FeCl2 aqueous solution (2 mg[Fe] mL−1) at room temperature. The resulting suspension then was stirred for 7 h. The Rh0 nanoparticles immersed in Fe2+ solution and parent Rh0 nanoparticles were also diluted in SiO2 as the reference samples (Rh loading of 0.5%) for handling and catalytic testing.
Reaction of Rh0 nanoparticles and Fe3+ cations
After washing three times with deionized, 1.25 mg Rh0 nanoparticles were dispersed in 4 mL deionized water and mixed with 1 mL FeCl3 aqueous solution (2 mg[Fe] mL−1) at room temperature. The resulting suspension then was stirred for 7 h.
Characterization
HAADF-STEM measurements were conducted with an aberration-corrected FEI Titan 80-300 STEM operated at 300 kV. EELS mapping and analysis were performed with aberration-corrected JEOL-ARM200F instrument operated at 200 kV. The instrument (Quantum 965) is capable of performing dual EELS experiment. The EELS mapping was performed in the STEM mode in the range of –50 to 500 eV for the zero-loss peak, 300 to 800 eV for the iron signal, and 2500 to 3500 eV for rhodium the signal maps. The zero-loss peak for zero-loss calibration was acquired in low loss spectrum images and aligned at 0 eV. The images and EELS data were analyzed and processed using Gatan Digital Micrograph software. The EELS maps were constructed by analyzing the Fe L2,3 (~708 eV), Rh L2,3 (~3004 eV), and Pt M4,5 (~2122 eV) edge peaks after the background subtraction.
X-ray absorption spectroscopy measurements were conducted in sector 20 of the Advanced Photon Source operated by Argonne National Laboratory. A rejection mirror was used to reduce the effects of harmonics. The metal foil was placed downstream of the sample cell, as a reference to calibrate the photon energy of each spectrum. The EXAFS spectra were analyzed with the ATHENA (χ(k) oscillation background removal), FEFF9 (theoretical model calculation), and ARTEMIS software packages. The fits to the Rh K-edge EXAFS χ(k) data were weighted by k2 and windowed between 1.5 Å−1 < k < 15.0 Å−1 using a Hanning window with dk = 1.0 Å−1.
H2 and CO2 chemisorption experiments were conducted with a Micromeritics 2020 instrument. In a typical procedure, 100 to 200 mg of the sample was degassed at 100 °C for 30 min, followed by in situ treatment at 200 °C in H2 and evacuation at 200 °C for 30 min. Then, the temperature was decreased to 35 °C under vacuum. Prior to the chemisorption experiments, the sample was further evacuated for 40 min. The adsorbates (H2 or CO2) were introduced into the system for the measurements of chemisorption isotherms. The first chemisorption isotherm was measured in the pressure range of 0–40 kPa at 35 °C. The sample was evacuated after the first adsorption cycle and a second chemisorption isotherm was recorded. The CO2 uptake on the parent Fe3O4 has been subtracted for plotting and derivation of adsorption parameters.
N2 physisorption experiments at −196 °C were performed on a Micromeritics 2020 instrument. The samples were degassed in vacuum at 200 °C before the measurements.
TOF-SIMS was applied with a TOF-SIMS V spectrometer (IONTOF GmbH, Münster, Germany) equipped with a 25 keV bismuth cluster ion source, a 20 keV Arn+, and a 2 keV Cs+/O2+ sputtering ion sources. Prior to the TOF-SIMS experiments, the samples were deposited on an Au(111) substrate and exposed to ultrahigh vacuum overnight.
X-ray diffraction experiments were performed in a Philips X′pert Multi-Purpose Diffractometer equipped with a Cu anode (50 kV and 40 mA).
The elemental composition of samples was measured by ICP optical emission spectroscopy (Perkin Elmer 7300DV). Prior to the ICP experiments, the samples were digested in a mixture of HNO3/HCl/HF/H2O followed by H3BO3 addition for extra HF treatment.
Reaction tests
The CO2 reduction was performed in a flow reactor equipped with an online gas chromatograph (Agilent 7890B). In a typical procedure, prior to the catalytic test, 12 mg of 30–80 mesh catalyst (diluted with 50 mg SiC) was loaded into the reactor and treated at 200 °C in 20 vol.% H2 with a ramping rate of 2 °C min−1. After the reactor reached the target reaction temperature, a mixture of CO2, H2, and He with a total flow rate of 140 mL min−1 was fed into the reactor (CO2:H2:He = 7:28:105).
Correlations of coordination number and metal dispersion
The correlation between coordination number and metal dispersion was derived from the data in the reference (Supplementary Fig. 12)35. The relationship between the coordination number of metal–metal shell and the metal dispersion was derived based on two different shapes of metal particles (spherical and raft-like shapes).
Calculation of adsorption constant and monolayer coverage from isotherms
The adsorption constant and monolayer coverage were derived from the chemisorption isotherms where chemisorption is treated as a chemical reaction between the gas-phase molecule (A) and the site (*) for adsorption (Eq. (5)).
The adsorption can be fitted with a Langmuir adsorption model (Eq. (6)).
The adsorption parameters can be obtained from the linear form of Eq. (2) (Eq. (7)). In Eq. (7), θA is the fractional coverage of the adsorption sites, P is the partial pressure of the adsorbate, Vm is the volume of the monolayer, and K is the equilibrium adsorption constant.
Data availability
The source data underlying Figs. 1–4 are provided as a Source Data file. The other relevant data that support the findings of this study are available from the corresponding author upon request. Source Data are provided with this paper.
References
Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345, 546–550 (2014).
Kattel, S., Liu, P. & Chen, J. G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 139, 9739–9754 (2017).
Kattel, S., Ramírez, P. J., Chen, J. G., Rodriguez, J. A. & Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355, 1296–1299 (2017).
Holewinski, A., Idrobo, J.-C. & Linic, S. High-performance Ag–Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 6, 828 (2014).
Calle-Vallejo, F. et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science 350, 185–189 (2015).
Matsubu, J. C., Yang, V. N. & Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 137, 3076–3084 (2015).
Zhao, M. et al. Ru octahedral nanocrystals with a face-centered cubic structure, {111} facets, thermal stability up to 400 °C, and enhanced catalytic activity. J. Am. Chem. Soc. 141, 7028–7036 (2019).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 100, 170–175 (1978).
Haller, G. L. & Resasco, D. E. Metal–support interaction: group VIII metals and reducible oxides. In Eley, D. D., Pines, H. & Weisz P. B. (eds) Advances in Catalysis. (Academic Press, 1989).
Dulub, O., Hebenstreit, W. & Diebold, U. Imaging cluster surfaces with atomic resolution: the strong metal-support interaction state of Pt supported on TiO2(110). Phys. Rev. Lett. 84, 3646–3649 (2000).
Tang, H. et al. Ultrastable hydroxyapatite/titanium-dioxide-supported gold nanocatalyst with strong metal–support interaction for carbon monoxide oxidation. Angew. Chem. Int. Ed. 55, 10606–10611 (2016).
Matsubu, J. C. et al. Adsorbate-mediated strong metal-support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Senanayake, S. D., Stacchiola, D. & Rodriguez, J. A. Unique properties of ceria nanoparticles supported on metals: novel inverse ceria/copper catalysts for CO oxidation and the water-gas shift reaction. Acc. Chem. Res. 46, 1702–1711 (2013).
Rodriguez, J. A. et al. Inverse oxide/metal catalysts in fundamental studies and practical applications: a perspective of recent developments. J. Phys. Chem. Lett. 7, 2627–2639 (2016).
Zhang, J. & Medlin, J. W. Catalyst design using an inverse strategy: from mechanistic studies on inverted model catalysts to applications of oxide-coated metal nanoparticles. Surf. Sci. Rep. 73, 117–152 (2018).
Cao, L. et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 565, 631–635 (2019).
Sun, Y. & Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
Zhang, H., Watanabe, T., Okumura, M., Haruta, M. & Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 11, 49–52 (2012).
Xia, X., Wang, Y., Ruditskiy, A. & Xia, Y. Galvanic replacement: a simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 25, 6313–6333 (2013).
Oh, M. H. et al. Galvanic replacement reactions in metal oxide nanocrystals. Science 340, 964–968 (2013).
An, K. & Hyeon, T. Synthesis and biomedical applications of hollow nanostructures. Nano Today 4, 359–373 (2009).
Cobley, C. M. & Xia, Y. Engineering the properties of metal nanostructures via galvanic replacement reactions. Mater. Sci. Eng. R. Rep. 70, 44–62 (2010).
González, E., Arbiol, J. & Puntes, V. F. Carving at the nanoscale: sequential galvanic exchange and kirkendall growth at room temperature. Science 334, 1377–1380 (2011).
Kim, K. W., Kim, S. M., Choi, S., Kim, J. & Lee, I. S. Electroless Pt deposition on Mn3O4 nanoparticles via the galvanic replacement process: electrocatalytic nanocomposite with enhanced performance for oxygen reduction reaction. ACS Nano 6, 5122–5129 (2012).
Sinatra, L. et al. A Au/Cu2O–TiO2 system for photo-catalytic hydrogen production. A pn-junction effect or a simple case of in situ reduction? J. Catal. 322, 109–117 (2015).
Peng, H. et al. Reversible Fe(ii) uptake/release by magnetite nanoparticles. Environ. Sci 5, 1545–1555 (2018).
Viollier, E., Inglett, P. W., Hunter, K., Roychoudhury, A. N. & Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem. 15, 785–790 (2000).
Pearce, C. I. et al. Synthesis and properties of titanomagnetite (Fe3−xTixO4) nanoparticles: a tunable solid-state Fe(II/III) redox system. J. Colloid Interface Sci. 387, 24–38 (2012).
Hakeem, A. A. et al. The role of rhodium in the mechanism of the water–gas shift over zirconia supported iron oxide. J. Catal. 313, 34–45 (2014).
Amoyal, M., Vidruk-Nehemya, R., Landau, M. V. & Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 348, 29–39 (2017).
Utsis, N., Landau, M. V., Erenburg, A., Nehemya, R. V. & Herskowitz, M. Performance of reverse water gas shift on coprecipitated and C-templated BaFe-hexaaluminate: the effect of Fe loading, texture, and promotion with K. ChemCatChem 10, 3795–3805 (2018).
Chou, C.-Y., Loiland, J. A. & Lobo, R. F. Reverse water-gas shift iron catalyst derived from magnetite. Catalysts 9, 773 (2019).
Chen, G. et al. Interfacial effects in iron-nickel hydroxide–platinum nanoparticles enhance catalytic oxidation. Science 344, 495–499 (2014).
Kip, B. J., Duivenvoorden, F. B. M., Koningsberger, D. C. & Prins, R. Determination of metal particle size of highly dispersed Rh, Ir, and Pt catalysts by hydrogen chemisorption and EXAFS. J. Catal. 105, 26–38 (1987).
Acknowledgements
This work is supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES), Division of Chemical Sciences, Geosciences and Biosciences (Transdisciplinary Approaches to Realize Novel Catalytic Pathways to Energy Carriers, FWP 47319). K.M.R. acknowledges support from the DOE BES Geosciences program at Pacific Northwest National Laboratory (PNNL) (Fundamental Mechanisms of Reactivity at Complex Geochemical Interfaces, FWP 56674). Portions of this work were performed at the William R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at PNNL. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, and was supported by the U.S. DOE (under Contract No. DE-AC02-06CH11357) and the Canadian Light Source and its funding partners. XAS spectra were taken with the help of Dr. Mahalingam Balasubramanian. We acknowledge help from PNNL colleagues Prof. Johannes Lercher, Dr. Janos Szanyi, and Dr. Zihua Zhu.
Author information
Authors and Affiliations
Contributions
Y.Z. and O.Y.G. led the project and conceived the experiments. Y.Z., X.Z., and K.K. performed the material synthesis. L.K. was responsible for the microscopy studies. J.L.F. was responsible for the X-ray absorption spectroscopy studies. K.M.R. contributed to the analysis of the mechanism and manuscript writing. Y.Z. and O.Y.G. wrote the manuscript with the inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Miron Landau and other, anonymous, reviewers for their contributions to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, Y., Zhang, X., Koh, K. et al. Inverse iron oxide/metal catalysts from galvanic replacement. Nat Commun 11, 3269 (2020). https://doi.org/10.1038/s41467-020-16830-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-16830-4
This article is cited by
-
Copper nanoparticles encapsulated in zeolitic imidazolate framework-8 as a stable and selective CO2 hydrogenation catalyst
Nature Communications (2024)
-
Engineered interfaces for heterostructured intermetallic nanomaterials
Nature Synthesis (2023)
-
Interfacial synergistic effect in SnO2/PtNi nanocrystals enclosed by high-index facets for high-efficiency ethylene glycol electrooxidation
Nano Research (2022)
-
XANES Investigations on Electronic Structure and Magnetic Properties of GaFeO3 Nanocrystals
Journal of Electronic Materials (2022)
-
Copper-zirconia interfaces in UiO-66 enable selective catalytic hydrogenation of CO2 to methanol
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.