Challenges for Microelectronics in Non-Invasive Medical Diagnostics
Abstract
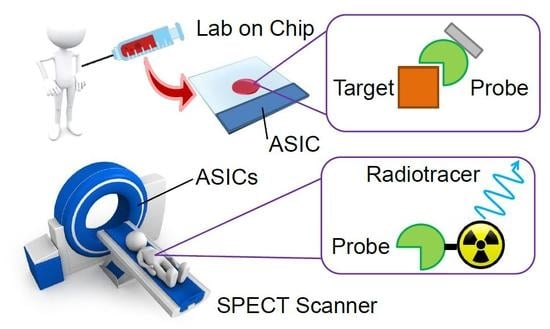
:1. Introduction
2. Lab-on-Chip Diagnostics
2.1. Circuit Challenges: Noise
2.2. Packaging Challenges
3. Multi-Modal Medical Imaging
3.1. PET
3.2. SPECT
4. SPECT Module Challenges and Outlook
4.1. Mutual Compatibility with MRI
4.2. Smaller, Faster Operation
4.3. Integrated Processing
4.4. Integration with Photonics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snoeys, W.; Anelli, G.; Campbell, M.; Cantatore, E.; Faccio, F.; Heijne, E.H.M.; Jarron, P.; Kloukinas, K.C.; Marchioro, A.; Moreira, P.; et al. Integrated circuits for particle physics experiments. IEEE J. Solid-State Circuits 2000, 35, 2018–2030. [Google Scholar] [CrossRef]
- Jeffrey, S.S.; Toner, M. Liquid biopsy: A perspective for probing blood for cancer. Lab Chip 2019, 19, 548–549. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Mulberry, G.; Smith, J.; Lindau, M.; Minch, B.A.; Sugaya, K.; Kim, B.N. Single-Cell Recording of Vesicle Release from Human Neuroblastoma Cells Using 1024-ch Monolithic CMOS Bioelectronics. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Viswam, V.; Bounik, R.; Shadmani, A.; Dragas, J.; Urwyler, C.; Boos, J.A.; Obien, M.E.J.; Muller, J.; Chen, Y.; Hierlemann, A. Impedance Spectroscopy and Electrophysiological Imaging of Cells with a High-Density CMOS Microelectrode Array System. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, H.; Mun, Y.; Ko, Y.; Cho, D.-I.D.; Ko, H. Low-Power and Low-Noise Capacitive Sensing IC Using Opamp Sharing Technique. IEEE Sensors J. 2016, 16, 7839–7840. [Google Scholar] [CrossRef]
- Rezaei, M.; Maghsoudloo, E.; Bories, C.; De Koninck, Y.; Gosselin, B. A Low-Power Current-Reuse Analog Front-End for High-Density Neural Recording Implants. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 271–280. [Google Scholar] [CrossRef]
- Mondal, S.; Hall, D.A. A 13.9-nA ECG Amplifier Achieving 0.86/0.99 NEF/PEF Using AC-Coupled OTA-Stacking. IEEE J. Solid-State Circuits 2020, 55, 414–425. [Google Scholar] [CrossRef]
- McFarlane, N.; Abshire, P. Comparative analysis of information rates of simple amplifier topologies. In Proceedings of the 2011 IEEE International Symposium of Circuits and Systems (ISCAS), Rio de Janeiro, Brazil, 15–18 May 2011; pp. 785–788. [Google Scholar]
- Tang, W.; Furth, P.M.; Nammi, V.H.; Panwar, G.; Ibarra, V.; Tang, X.; Unguez, G.A.; Misra, S. An Aquatic Wireless Biosensor for Electric Organ Discharge with an Integrated Analog Front End. IEEE Sensors J. 2019, 19, 6260–6269. [Google Scholar] [CrossRef]
- Hafizh, I.; Carminati, M.; Fiorini, C. Assessment of analog pulse processor performance for ultra high-rate x-ray spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. A 2019, 945, 162479. [Google Scholar] [CrossRef]
- Zhao, X.; Sadhu, V.; Le, T.; Pompili, D.; Javanmard, M. Toward Wireless Health Monitoring via an Analog Signal Compression-Based Biosensing Platform. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 461–470. [Google Scholar] [CrossRef]
- Tang, W.; Culurciello, E. A Low-Power High-Speed Ultra-Wideband Pulse Radio Transmission System. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Crescentini, M.; Bennati, M.; Carminati, M.; Tartagni, M. Noise Limits of CMOS Current Interfaces for Biosensors: A Review. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Azzellino, G.; Ragni, A.; Carminati, M.; Ferrari, G. Resonant noise-canceling current front-end for high-resolution impedance sensing. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–6. [Google Scholar]
- Hartmann, R.; Hauff, D.; Krisch, S.; Lechner, P.; Lutz, G.; Richter, R.H.; Seitz, H.; Struder, L.; Bertuccio, G.; Fasoli, L.; et al. Design and test at room temperature of the first silicon drift detector with on-chip electronics. In Proceedings of the 1994 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 11–14 December 1994; pp. 535–538. [Google Scholar]
- Carminati, M.; Annoni, A.; Morichetti, F.; Guglielmi, E.; Ferrari, G.; de Aguiar, D.O.M.; Melloni, A.; Sampietro, M. Design Guidelines for Contactless Integrated Photonic Probes in Dense Photonic Circuits. J. Lightwave Technol. 2017, 35, 3042–3049. [Google Scholar] [CrossRef]
- Carminati, M.; Ferrari, G.; Vahey, M.D.; Voldman, J.; Sampietro, M. Miniaturized Impedance Flow Cytometer: Design Rules and Integrated Readout. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.; Gascón, D.; Fernández, G.; Sanuy, A.; Mauricio, J.; Graciani, R.; Sanchez, D. MUSIC: An 8 channel readout ASIC for SiPM arrays. In Optical Sensing and Detection IV; Berghmans, F., Mignani, A.G., Eds.; SPIE Photonics Europe: Brussels, Belgium, 2016; p. 98990G. [Google Scholar]
- Carminati, M. Advances in High-Resolution Microscale Impedance Sensors. J. Sens. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Schlecker, B.; Hoffmann, A.; Chu, A.; Ortmanns, M.; Lips, K.; Anders, J. Towards Low-Cost, High-Sensitivity Point-of-Care Diagnostics Using VCO-Based ESR-on-a-Chip Detectors. IEEE Sens. J. 2019, 19, 8995–9003. [Google Scholar] [CrossRef]
- Handwerker, J.; Schlecker, B.; Ortmanns, M.; Anders, J. Integrated Circuit Technology for Next Generation Point-of-Care Spectroscopy Applications. IEEE Commun. Mag. 2017, 55, 143–151. [Google Scholar] [CrossRef]
- Piedimonte, P.; Fucile, S.; Limatola, C.; Renzi, M.; Palma, F. Biocompatibility of Silicon Nanowires: A Step towards IC Detectors; AIP Conference Proceedings: Fes, Morocco, 2019; p. 020011. [Google Scholar]
- Ciccarella, P.; Carminati, M.; Sampietro, M.; Ferrari, G. Multichannel 65 zF rms Resolution CMOS Monolithic Capacitive Sensor for Counting Single Micrometer-Sized Airborne Particles on Chip. IEEE J. Solid-State Circuits 2016, 51, 2545–2553. [Google Scholar] [CrossRef]
- Rosenstein, J.K.; Wanunu, M.; Merchant, C.A.; Drndic, M.; Shepard, K.L. Integrated nanopore sensing platform with sub-microsecond temporal resolution. Nat Methods 2012, 9, 487–492. [Google Scholar] [CrossRef]
- Forouhi, S.; Dehghani, R.; Ghafar-Zadeh, E. CMOS based capacitive sensors for life science applications: A review. Sens. Actuators A Phys. 2019, 297, 111531. [Google Scholar] [CrossRef]
- Carminati, M.; Gervasoni, G.; Sampietro, M.; Ferrari, G. Note: Differential configurations for the mitigation of slow fluctuations limiting the resolution of digital lock-in amplifiers. Rev. Sci. Instrum. 2016, 87, 026102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, J.-C.; Niknejad, A.M. Oscillator-Based Reactance Sensors With Injection Locking for High-Throughput Flow Cytometry Using Microwave Dielectric Spectroscopy. IEEE J. Solid-State Circuits 2016, 51, 457–472. [Google Scholar] [CrossRef]
- Hadizadeh, R.; Laitinen, A.; Molinero, D.; Pereira, N.; Pinheiro, M. Wafer-Level Fan-Out For High-Performance, Low-Cost Packaging Of Monolithic Rf Mems/Cmos. In Proceedings of the 2018 International Wafer Level Packaging Conference (IWLPC), San Jose, CA, USA, 23–25 October 2018; pp. 1–6. [Google Scholar]
- Thomson, D.; Zilkie, A.; Bowers, J.E.; Komljenovic, T.; Reed, G.T.; Vivien, L.; Marris-Morini, D.; Cassan, E.; Virot, L.; Fédéli, J.-M.; et al. Roadmap on silicon photonics. J. Opt. 2016, 18, 073003. [Google Scholar] [CrossRef]
- Huang, Y.; Mason, A.J. Lab-on-CMOS integration of microfluidics and electrochemical sensors. Lab Chip 2013, 13, 3929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta-Chaudhuri, T.; Smela, E.; Abshire, P.A. System-on-Chip Considerations for Heterogeneous Integration of CMOS and Fluidic Bio-Interfaces. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 1129–1142. [Google Scholar] [CrossRef] [Green Version]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Raia, L.; Rondelli, N.; Bianchessi, M.; Carminati, M. Microfluidic structures for large-scale manufacture combining photo-patternable materials. RSC Adv. 2016, 6, 59155–59159. [Google Scholar] [CrossRef]
- Jiang, W.; Chalich, Y.; Deen, M.J. Sensors for Positron Emission Tomography Applications. Sensors 2019, 19, 5019. [Google Scholar] [CrossRef] [Green Version]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner*. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef] [PubMed]
- The 10-ps Challenge. Available online: https://the10ps-challenge.org (accessed on 26 June 2020).
- Gundacker, S.; Turtos, R.M.; Auffray, E.; Paganoni, M.; Lecoq, P. High-frequency SiPM readout advances measured coincidence time resolution limits in TOF-PET. Phys. Med. Biol. 2019, 64, 055012. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; de la Taille, C.; Fleury, J.; Seguin-Moreau, N.; Raux, L.; Callier, S.; Martin-Chassard, G.; Dulucq, F.; Thienpont, D. Triroc, a versatile 64-channel SiPM readout ASIC for time-of-flight PET. In Proceedings of the 2016 IEEE Nuclear Science Symposium, Medical Imaging Conference and Room-Temperature Semiconductor Detector Workshop (NSS/MIC/RTSD), Strasbourg, France, 29 October–6 November 2016; pp. 1–5. [Google Scholar]
- Shen, W.; Harion, T.; Chen, H.; Briggl, K.; Stankova, V.; Munwes, Y.; Schultz-Coulon, H.-C. A Silicon Photomultiplier Readout ASIC for Time-of-Flight Applications Using a New Time-of-Recovery Method. IEEE Trans. Nucl. Sci. 2018, 65, 1196–1202. [Google Scholar] [CrossRef]
- Sarasola, I.; Nemallapudi, M.V.; Gundacker, S.; Sánchez, D.; Gascón, D.; Rato, P.; Marín, J.; Auffray, E. A comparative study of the time performance between NINO and FlexToT ASICs. J. Inst. 2017, 12, P04016. [Google Scholar] [CrossRef]
- Trigilio, P.; Busca, P.; Quaglia, R.; Occhipinti, M.; Fiorini, C. A SiPM-Readout ASIC for SPECT Applications. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 404–410. [Google Scholar] [CrossRef]
- Occhipinti, M.; Carminati, M.; Busca, P.; Butt, A.D.; Montagnani, G.L.; Trigilio, P.; Piemonte, C.; Ferri, A.; Gola, A.; Bukki, T.; et al. Characterization of the Detection Module of the INSERT SPECT/MRI Clinical System. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 554–563. [Google Scholar] [CrossRef]
- Carminati, M.; Valtorta, S.; Belloli, S.; Moresco, R.M.; Savi, A.; Iadanza, A.; Falini, A.; Politi, L.S.; Cadioli, M.; Hutton, B.F.; et al. Validation and Performance Assessment of a Preclinical SiPM-Based SPECT/MRI Insert. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 483–490. [Google Scholar] [CrossRef]
- Carminati, M.; D’Adda, I.; Morahan, A.J.; Erlandsson, K.; Nagy, K.; Czeller, M.; Tolgyesi, B.; Nyitrai, Z.; Savi, A.; van Mullekom, P.; et al. Clinical SiPM-Based MRI-Compatible SPECT: Preliminary Characterization. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 371–377. [Google Scholar] [CrossRef]
- Carminati, M.; Montagnani, G.L.; Occhipinti, M.; Kuehne, A.; Niendorf, T.; Nagy, K.; Nagy, A.; Czeller, M.; Fiorini, C. SPECT/MRI INSERT Compatibility: Assessment, Solutions, and Design Guidelines. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 369–379. [Google Scholar] [CrossRef]
- Marques, J.P.; Simonis, F.F.J.; Webb, A.G. Low-field MRI: An MR physics perspective. J. Magn. Reson. Imaging 2019, 49, 1528–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, L.A.; Pedemonte, S.; DeWitt, D.; MacDonald, L.; Hunter, W.C.J.; Van Leemput, K.; Miyaoka, R. Characterization of highly multiplexed monolithic PET / gamma camera detector modules. Phys. Med. Biol. 2018, 63, 075017. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, L.; Vita, D.D.; Minerva, A.; Carminati, M.; Fiorini, C. A SiPM-Based Directional Gamma-Ray Spectrometer with Embedded Machine Learning. In Proceedings of the 2020 2nd IEEE International Conference on Artificial Intelligence Circuits and Systems (AICAS), Genova, Italy, 31 August–2 September 2020; pp. 168–172. [Google Scholar]
- Sun, Y.; Cheng, A.C. Machine learning on-a-chip: A high-performance low-power reusable neuron architecture for artificial neural networks in ECG classifications. Comput. Biol. Med. 2012, 42, 751–757. [Google Scholar] [CrossRef]
- Seo, J.; Lin, B.; Kim, M.; Chen, P.-Y.; Kadetotad, D.; Xu, Z.; Mohanty, A.; Vrudhula, S.; Yu, S.; Ye, J.; et al. On-Chip Sparse Learning Acceleration With CMOS and Resistive Synaptic Devices. IEEE Trans. Nanotechnol. 2015, 14, 969–979. [Google Scholar] [CrossRef]
- Basu, A.; Acharya, J.; Karnik, T.; Liu, H.; Li, H.; Seo, J.-S.; Song, C. Low-Power, Adaptive Neuromorphic Systems: Recent Progress and Future Directions. IEEE J. Emerg. Sel. Topics Circuits Syst. 2018, 8, 6–27. [Google Scholar] [CrossRef]
- Alimonti, G.; Ammendola, R.; Andreazza, A.; Badoni, D.; Bonaiuto, V.; Casalboni, M.; De Matteis, F.; Mai, A.; Paoluzzi, G.; Prosposito, P.; et al. Use of silicon photonics wavelength multiplexing techniques for fast parallel readout in high energy physics. Nucl. Instrum. Methods Phys. Res. Sect. A 2019, 936, 601–603. [Google Scholar] [CrossRef]
- Allam, M.; Cai, S.; Ganesh, S.; Venkatesan, M.; Doodhwala, S.; Song, Z.; Hu, T.; Kumar, A.; Heit, J.; Study Group, C.; et al. COVID-19 Diagnostics, Tools, and Prevention. Diagnostics 2020, 10, 409. [Google Scholar] [CrossRef]








| Parameter | Preclinical [45] | Clinical [46] |
|---|---|---|
| Number of detection modules | 10 | 20 |
| Number of pixels | 360 | 1440 |
| Inner bore diameter [cm] | 4 | 33 |
| Outer insert diameter [cm] | 20 | 50 |
| Scintillator size [cm × cm × cm] | 5 × 5 × 0.8 | 10 × 5 × 0.8 |
| Energy resolution FWHM @140 keV | 12% | 16% |
| Extrinsic spatial resolution [mmFWHM] | 0.9 | 10 |
| Sensitivity [cps/MBq] | 1105 | 365 |
| Trans-axial Field of View [mm] | 15.6 | 200 |
| Collimator type | Multi-Pinhole | MM Slit-Slat |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carminati, M.; Fiorini, C. Challenges for Microelectronics in Non-Invasive Medical Diagnostics. Sensors 2020, 20, 3636. https://doi.org/10.3390/s20133636
Carminati M, Fiorini C. Challenges for Microelectronics in Non-Invasive Medical Diagnostics. Sensors. 2020; 20(13):3636. https://doi.org/10.3390/s20133636
Chicago/Turabian StyleCarminati, Marco, and Carlo Fiorini. 2020. "Challenges for Microelectronics in Non-Invasive Medical Diagnostics" Sensors 20, no. 13: 3636. https://doi.org/10.3390/s20133636