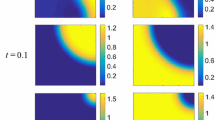
Abstract
Small-scale spatial variability can affect community dynamics in many ecological and biological processes, such as predator-prey dynamics and immune responses. Spatial variability includes short-range neighbour-dependent interactions and small-scale spatial structure, such as clustering where individuals aggregate together, and segregation where individuals are spaced apart from one another. Yet, a large class of mathematical models aimed at representing these processes ignores these factors by making a classical mean-field approximation, where interactions between individuals are assumed to occur in proportion to their average density. Such mean-field approximations amount to ignoring spatial structure. In this work, we consider an individual-based model of a two-species community that is composed of consumers and resources. The model describes migration, predation, competition and dispersal of offspring, and explicitly gives rise to varying degrees of spatial structure. We compare simulation results from the individual-based model with the solution of a classical mean-field approximation, and this comparison provides insight into how spatial structure can drive the system away from mean-field dynamics. Our analysis reveals that mechanisms leading to intraspecific clustering and interspecific segregation, such as short-range predation and short-range dispersal, tend to increase the size of the resource species relative to the mean-field prediction. We show that under certain parameter regimes these mechanisms lead to the extinction of consumers whereas the classical mean-field model predicts the coexistence of both species.








Similar content being viewed by others
References
Abrams PA (2000) The evolution of predator-prey interactions: theory and evidence. Annual Rev Ecol Syst 31:79–105
Abrams PA, Ginzburg LR (2000) The nature of predation: prey dependent, ratio dependent or neither?. Trends Ecol Evol 15:337–341
Agnew DJG, Green JEF, Brown TM, Simpson MJ, Binder BJ (2014) Distinguishing between mechanisms of cell aggregation using pair-correlation functions. J Theor Biol 352:16–23
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Baker RE, Simpson MJ (2010) Correcting mean-field approximations for birth-death-movement processes. Phys Rev E 82:041905
Barraquand F, Murrell DJ (2012) Intense or spatially heterogeneous predation can select against prey dispersal. PLoS One 7:e28924
Binder BJ, Simpson MJ (2015) Spectral analysis of pair-correlation bandwidth: application to cell biology images. Royal Soc Open Sci 2:140494
Binny RN, Haridas P, James A, Law R, Simpson MJ, Plank MJ (2016a) Spatial structure arising from neighbour-dependent bias in collective cell movement. PeerJ 4:e1689
Binny RN, James A, Plank MJ (2016b) Collective cell behaviour with neighbour-dependent proliferation, death and directional bias. Bull Math Biol 78:2277–2301
Binny RN, Law R, Plank MJ (2020) Living in groups: Spatial-moment dynamics with neighbour-biased movements. Ecol Model 415:108825
Binny RN, Plank MJ, James A (2015) Spatial moment dynamics for collective cell movement incorporating a neighbour-dependent directional bias. J R Soc Interface 12:20150228
Bolker BM, Pacala SW (1999) Spatial moment equations for plant competition: understanding spatial strategies and the advantages of short dispersal. Am Natur 153:575–602
Brigatti E, Oliva M, Nunez-Lopez M, Oliveros-Ramos R, Benavides J (2009) Pattern formation in a predator-prey system characterized by a spatial scale of interaction. Europhys Lett 88:68002
Britton N (2003) Essential mathematical biology. Springer, London
Browning AP, McCue SW, Binny RN, Plank MJ, Shah ET, Simpson MJ (2018) Inferring parameters for a lattice-free model of cell migration and proliferation using experimental data. J Theor Biol 437:251–260
Browning AP, Jin W, Plank MJ, Simpson MJ (2020) Identifying density-dependent interactions in collective cell behaviour. J R Soc Interface 17:20200143.
Cantrell RS, Cosner C (2004) Deriving reaction–diffusion models in ecology from interacting particle systems. J Math Biol 48:187–217
Cuddington KM, Yodzis P (2000) Diffusion-limited predator-prey dynamics in euclidean environments: an allometric individual-based model. Theor Popul Biol 58:259–278
Dini S, Binder BJ, Green JEF (2018) Understanding interactions between populations: individual based modelling and quantification using pair correlation functions. J Theor Biol 439:50–64
Dobramysl U, Tauber UC (2013) Environmental versus demographic variability in two-species predator-prey models. Phys Rev Lett 110:048105
Edelstein-Keshet L (2005) Mathematical models in biology (classics in applied mathematics). Society for Industrial and Applied Mathematics, New York
Fadai NT, Johnston ST, Simpson MJ (2019) Unpacking the Allee effect: determining individual-level mechanisms that drive population dynamics. https://doi.org/10.1101/774000v1https://doi.org/10.1101/774000v1bioRxiv.Accessed. December 2019
Galetti M, Moleon M, Jordano P, Pires MM, Guimaraes PR Jr, Pape T, Nichols E, Hansen D, Olesen JM, Munk M, de Mattos JS, Schweiger AH, Owen-Smith N, Johnson CN, Marquis RJ, Svenning JC (2018) Ecological and evolutionary legacy of megafauna extinctions. Biol Rev 93:845–862
Gerum R, Richter S, Fabry B, Bohec CL, Bonadonna F, Nesterova A, Zitterbart DP (2018) Structural organisation and dynamics in king penguin colonies. J Phys D:, Appl Phys 51:164004
Gillespie DT (1977) Exact stochastic simulation of coupled chemical reactions. J Phys Chem 81:2340–2361
Grunbaum D (2012) The logic of ecological patchiness. Inter Focus 2:150–155
Hillen T, Painter KJ (2009) A user’s guide to PDE models for chemotaxis. J Math Biol 58:183–217
Hosseini PR (2003) How localized consumption stabilizes predator-prey systems with finite frequency of mixing. Am Natur 161:567–585
Hosseini PR (2006) Pattern formation and individual-based models: the importance of understanding individual-based movement. Ecol Model 194:357–371
Hunt VM, Brown JS (2018) Coexistence and displacement in consumer-resource systems with local and shared resources. Theor Ecol 11:83–93
Jin W, McCue SW, Simpson MJ (2018) Extended logistic growth model for heterogeneous populations. J Theor Biol 445:51–61
Johnston ST, Baker RE, McElwain DLS, Simpson MJ (2017) Co-operation, competition and crowding: a discrete framework linking Allee kinetics, nonlinear diffusion, shocks and sharp-fronted travelling waves. Sci Report 7:42134
Kuperman MN, Laguna MF, Abramson G, Monjeau JA (2019) Meta-population oscillations from satiation of predators. Phys A 527:121288
Law R, Dieckmann U (2000) A dynamical system for neighbourhoods in plant communities. Ecology 81:2137–2148
Law R, Illian J, Burslem DFRP, Gratzer G, Gunatilleke CVS, Gunatilleke IAUN (2009) Ecological information from spatial patterns of plants: insights from point process theory. J Ecol 97:616–628
Law R, Murrell DJ, Dieckmann U (2003) Population growth in space and time: spatial logistic equations. Ecology 84:252–262
Markham DC, Simpson MJ, Maini PK, Gaffney EA, Baker RE (2013) Incorporating spatial correlations into multispecies mean-field models. Phys Rev E 88:052713
Mathworks (2019) https://www.mathworks.com/help/matlab/ref/ode45.htmlhttps://www.mathworks.com/help/matlab/ref/ode45.html Solve nonstiff differential equations — medium order method. Accessed December 2019
Mobilia M, Georgiev IT, Tauber UC (2006) Fluctuations and correlations in lattice models for predator-prey interaction. PhysRev E 73(R):040903
Mobilia M, Georgiev IT, Tauber UC (2007) Phase transitions and spatio-temporal fluctuations in stochastic lattice Lotka–Volterra models. J Statis Phys 128:447–483
Murray JD (1989) Mathematical biology. Springer, New York
Murrell DJ (2005) Local spatial structure and predator-prey dynamics: counterintuitive effects of prey enrichment. Am Natur 166:354–367
Ovaskainen O, Finkelshtein D, Kutoviy O, Cornell S, Bolker B, Kondratiev Y (2014) A general mathematical framework for the analysis of spatiotemporal point processes. Theor Ecol 7:101–113
Penczykowski RM, Laine A, Koskella B (2016) Understanding the ecology and evolution of host–parasite interactions across scales. Evol Appl 9:37–52
Plank MJ, Law R (2015) Spatial point processes and moment dynamics in the life sciences: a parsimonious derivation and some extensions. Bull Math Biol 77:586–613
Plank MJ, Simpson MJ, Binny RN (2019) Small-scale spatial structure influences large-scale invasion rates. Theoretical Ecology. https://doi.org/10.1007/s12080-020-00450-1
Rincon DF, Canas LA, Hoy CW (2017) Modeling changes in predator functional response to prey across spatial scales. Theor Ecol 10:403–415
Santora JA, Reiss CS, Loeb VJ, Veit RR (2010) Spatial association between hotspots of baleen whales and demographic patterns of Antarctic krill Euphausia superba suggests size-dependent predation. Mar Ecol Prog Ser 405:255–269
Soehnlein O, Steffens S, Hidalgo A, Weber C (2017) Neutrophils as protagonists and targets in chronic inflammation. Nature Rev Immunol 17:248–261
Stephens PA, Sutherland WJ, Freckleton RP (1999) What is the Allee effect?. Oikos 87:185–190
Surendran A, Plank MJ, Simpson MJ (2018) Spatial moment description of birth-death-movement processes incorporating the effects of crowding and obstacles. Bull Math Biol 80:2828–2855
Surendran A, Plank MJ, Simpson MJ (2019) Spatial structure arising from chase-escape interactions with crowding. Sci Report 9:14988
Tobin P, Bjornstad ON (2003) Spatial dynamics and cross-correlation in a transient predator-prey system. J Animal Ecol 72:460–467
Treloar KK, Simpson MJ, Binder BJ, McElwain DLS, Baker RE (2015) Assessing the role of spatial correlations during collective cell spreading. Sci Report 4:5713
Vijay K (2018) Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol 59:391–412
Wang H, Nagy JD, Gilg O, Kuang Y (2009) The roles of predator maturation delay and functional response in determining the periodicity of predator–prey cycles. Math Biosci 221:1–10
Wilson WG (1998) Resolving discrepancies between deterministic population models and individual-based simulations. Am Natur 151:116–134
Acknowledgements
We thank the Associate Editor and anonymous referees for their helpful suggestions.
Funding
This work is supported by the Australian Research Council (DP170100474). MJP is partly supported by Te Pūnaha Matatini, a New Zealand Centre of Research Excellence.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Data availability
MATLAB code used to generate results are available on Github at https://github.com/Anudeep-Surendran/Surendran2020
Rights and permissions
About this article
Cite this article
Surendran, A., Plank, M.J. & Simpson, M.J. Small-scale spatial structure affects predator-prey dynamics and coexistence. Theor Ecol 13, 537–550 (2020). https://doi.org/10.1007/s12080-020-00467-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-020-00467-6