Abstract
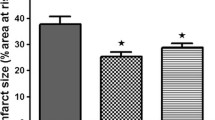
We recently reported that non-preconditioned hearts from diet-induced obese rats showed, compared to controls, a significant reduction in infarct size after ischaemia/reperfusion, whilst ischaemic preconditioning was without effect. In view of the high circulating FFA concentration in diet rats, the aims of the present study were to: (i) compare the effect of palmitate on the preconditioning potential of hearts from age-matched controls and diet rats (ii) elucidate the effects of substrate manipulation on ischaemic preconditioning. Substrate manipulation was done with dichloroacetate (DCA), which enhances glucose oxidation and decreases fatty acid oxidation. Isolated hearts from diet rats, age-matched controls or young rats, were perfused in the working mode using the following substrates: glucose (10 mM); palmitate (1.2 mM)/3% albumin) + glucose (10 mM) (HiFA + G); palmitate (1.2 mM/3% albumin) (HiFA); palmitate (0.4 mM/3% albumin) + glucose(10 mM) (LoFA + G); palmitate (0.4 mM/3% albumin) (LoFA). Hearts were preconditioned with 3 × 5 min ischaemia/reperfusion, followed by 35 min coronary ligation and 60 min reperfusion for infarct size determination (tetrazolium method) or 20 min global ischaemia/10 or 30 min reperfusion for Western blotting (ERKp44/42, PKB/Akt). Preconditioning of glucose-perfused hearts from age-matched control (but not diet) rats reduced infarct size, activated ERKp44/42 and PKB/Akt and improved functional recovery during reperfusion (ii) perfusion with HiFA + G abolished preconditioning and activation of ERKp44/42 (iii) DCA pretreatment largely reversed the harmful effects of HiFA. Hearts from non-preconditioned diet rats exhibited smaller infarcts, but could not be preconditioned, regardless of the substrate. Similar results were obtained upon substrate manipulation of hearts from young rats. Abolishment of preconditioning in diet rats may be due to altered myocardial metabolic patterns resulting from changes in circulating FA. The harmful effects of HiFA were attenuated by stimulation of glycolysis and inhibition of FA oxidation.













Similar content being viewed by others
References
Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A (2008) Ghaleh B Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol 295(4):H1580–H1586. https://doi.org/10.1152/ajpheart.00379.2008
du Toit EF, Smith W, Muller C, Strijdom H, Stouthammer B, Woodiwiss AJ, Norton GR, Lochner A (2008) Myocardial susceptibility to ischemic-reperfusion injury in a prediabetic model of dietary-induced obesity. Am J Physiol Heart Circ Physiol 294(5):H2336–H2343. https://doi.org/10.1152/ajpheart.00486.2007
Wensley I, Salaveria K, Bulmer AC, Donner DG, duToit EF (2013) Myocardial structure, function and ischemic tolerance in a rodent model of obesity with insulin resistance. Exp Physiol 98(11):1552–1564. https://doi.org/10.1113/expphysiol.2013.074948
Chase PJ, Davis PG, Bensimhon DR (2014) The obesity paradox in chronic heart failure: what does it mean? Curr Heart Fail Rep 11(1):11–17. https://doi.org/10.1007/s11897-013-0184-2
Clark AL, Fonarow GC, Horwich TB (2014) Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis 56(4):409–410. https://doi.org/10.1016/j.pcad.2013.10.004
Niedziela J, Hadzik B, Niedziela N, Gasior M et al (2014) The obesity paradox in acute coronary syndrome: a meta-analysis. Europ J Epidemiol 29(11):801–812. https://doi.org/10.1001/s10654-014-9661-9
Miki T, Itoh T, Sunaga D, Miura T (2012) Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 11:67. https://doi.org/10.1186/1475-2840-11-67
Whittington HJ, Babu GG, Mocanu NM, Yellon DM, Hausenloy DJ (2012) The diabetic heart: too sweet for its own good? Cardiol Res Pract 2012:845698. https://doi.org/10.1155/2012/845698
Clark C, Smith W, Lochner A, du Toit EF (2011) The effects of gender and obesity on myocardial tolerance to ischemia. Physiol Res 60(2):291–301
Nduhirabandi F, du Toit EF, Blackhurst D, Marais D, Lochner A (2011) Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischaemia and reperfusion injury in a pre-diabetic model of diet-induced obesity. J Pineal Res 50(2):171–192. https://doi.org/10.1111/j.1600-079x.2010.00826.x
Donner D, Headrick JP, Peart JN, du Toit EF (2013) Obesity improves myocardial ischemic tolerance and RISK signalling in insulin-insensitive rats. Dis Mod Mech 6(2):457–466. https://doi.org/10.1242/dmm.010959
Balakumar P, Sharma NK (2012) Healing the diabetic heart: does myocardial preconditioning work? Cell Signal 24(1):53–59. https://doi.org/10.1016/cellsig.2011.09.007
Lejay A, Fang F, John R, Van JAD, Barr M, Thaveau F, Chafke N, Geny B, Scholey JW (2016) Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol 91:11–22. https://doi.org/10.1016/yjmcc.2015.12020
Przyklenk K (2013) Reduction of myocardial infarct size with ischemic conditioning: physiologic and technical considerations. Anesth Analg 117(4):891–901. https://doi.org/10.1213/ANE.ob013e318294fc63
Kristiansen SB, Lofgren B, Stottrup NB, Lhatir D, Nielsen-Kudsk JE, Nielsen TT et al (2004) Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 47(10):1716–1721. https://doi.org/10.1007/s00125-004-1514-4
Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW (2007) Myocardial preconditioning against ischemia–reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol 292(2):R920–R926. https://doi.org/10.1152/ajpregu.00520.2006
Zhu SG, Xi L, Kukreja RC (2012) Type 2 diabetic obese db/db mice are refractory to myocardial ischaemic postconditioning in vivo: potential role for Hsp 20, F1-ATPase delta and Echs1. J Cell Mol Med 16(4):950–958. https://doi.org/10.1111/j.1582-4934.2011.01376.x
Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM et al (2013) Cardioprotection in the aging, diabetic heart: the loss of protective Akt signaling. Cardiovasc Res 99(4):694–704
Ji L, Zhang X, Liu W, Huang Q, Fu F, Ma H et al (2013) AMPK-regulated and Akt-dependent enhancement of glucose uptake is essential in ischemic preconditioning-alleviated reperfusion injury. PLoS ONE 8(7):e69910. https://doi.org/10.1371/journal.pone.0069910
Hadour G, Ferrera R, Sebbag L, Forrat R, Delaye J, de Lorgeril M (1998) Improved myocardial tolerance to ischaemia in the diabetic rabbit. J Mol Cell Cardiol 30(9):1869–1875. https://doi.org/10.1006/jmcc.1998.0751
Salie R, Huisamen B, Lochner A (2014) High carbohydrate and high fat diets protect the heart against ischaemia/reperfusion injury. Cardiovasc Diabetol 13:109. https://doi.org/10.1188/1475-2840-13-13
Song P, Wu Y, Xu J, Xie Z, Dong Y, Zhang M et al (2007) Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase angiotensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation 116(14):1585–1595. https://doi.org/10.1161/CICULATIONAHA,107.716498
Mocanu MN, Yellon DM (2007) PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? Br J Pharmacol 150(7):833–838. https://doi.org/10.1038/sj.bjp.0707155
Wang B, Raedschelders K, Shravah J, Hui Y, Safaei HG, Chen DD et al (2011) Differences in myocardial PTEN expression and Akt signaling in type 2 diabetic and nondiabetic patients undergoing coronary bypass surgery. Clin Endocrinol 74(6):705–713. https://doi.org/10.1111/j.1365-2265.2011.03979.x
Przyklenk K, Maynard M, Greiner DL, Whittaker P (2011) Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 14(5):781–790. https://doi.org/10.1089/ars.2010.3343
Przyklenk K (2011) Efficacy of cardioprotective conditioning strategies in aging and diabetic cohorts: the comorbidity conundrum. Drugs Aging 28(5):331–343. https://doi.org/10.2165/11587190.000000000.00000
Xia X, Zhao B, Wu Y, Hou JB, Zhang L, Xu JJ et al (2011) Ginsenoside Rb1 conditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J Biomed Biotechnol 2011:767930. https://doi.org/10.1155/2011/767930
Kim HS, Cho JE, Hwang KC, Shim YH, Lee JH, Kwak YL (2010) Diabetes mellitus mitigates cardioprotective effects of remifentanil preconditioning in ischemia-reperfused rat hearts in association with antiapoptotic pathways of survival. Eur J Pharmacol 628(1–3):132–139. https://doi.org/10.1016/j.ejphar.2009.11.032
Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H (2007) Western diet but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406(3):457–467. https://doi.org/10.1042/BJ20070392
Harmancey R, Vasquez HG, Guthrie PH, Taegtmeyer H (2013) Decreased long-chain fatty acid oxidation impairs postischemic recovery of the insulin-resistant rat heart. FASEB J 27(10):3966–3978. https://doi.org/10.1096/fj.13-234914
Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED (2004) Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53(9):2366–2374. https://doi.org/10.2337/diabetes.53.9.2366
Buchanan J, Mazumder PK, Ping H, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED (2005) Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146(12):5341–5349. https://doi.org/10.1210/en.2005-0938
Opie LH (1998) The Heart. Physiology, from cell to circulation. Chapter 11. Lippincott-Raven Publishers, Philadelphia
Lopaschuk G (2000) Regulation of carbohydrate metabolism in ischemia and reperfusion. Am Heart J 139(2 Pt 3):5115–5119. https://doi.org/10.1067/mhj.2000.103919
Van der Vusse GJ, Van Bilsen M (2006) Free fatty acids and postischemic myocardial function. Semin Cardiothorac Vasc Anesth 10(3):231–235. https://doi.org/10.1177/1089253206291319
Carroll R, Carley AN, Dyck JR, Severson DL (2005) Metabolic effects of insulin on cardiomyocytes from control and diabetic db/db mouse hearts. Am J Physiol Endocrinol Metab 288:E900–E906. https://doi.org/10.1152/ajpendo.DD491.2004
Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, Larsen TS (2002) Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol. 283(3):H949–H957. https://doi.org/10.1152/ajpheart.00226.2001
Belke DD, Larsen TS, Gibbs EM, Severson DL (2000) Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 279(5):E1104–E1113. https://doi.org/10.1152/ajpendo.2000.27915.E1104
Oliver MF, Yates PA (1971) Induction of ventricular arrhythmias by elevation of arterial free fatty acids in experimental myocardial infarction. Cardiology 56(1):359–364. https://doi.org/10.1159/0001.169385
Oliver MF, Opie LH (1994) Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet 343(8890):155–158. https://doi.org/10.1016/s0140-6736(94)90939-3
Kurien VA, Yates PA, Oliver MF (1971) The role of free fatty acids in the production of ventricular arrhythmias after acute coronary artery occlusion. Eur J Clin Invest 1(4):225–241. https://doi.org/10.1111/eci.1976.4.225
Liedtke AJ, DeMaison L, Eggelston AM, Cohen LM, Nellis SH (1988) Changes in substrate metabolism and effects of excess fatty acids in reperfused myocardium. Circ Res 62(3):535–542. https://doi.org/10.1161/01.res.62.3.535
Lopaschuk GD, Spafford MA, Davies NJ, Wall SJ (1990) Glucose and palmitate oxidation in isolated working rat hearts reperfused after a period of transient global ischemia. Circ Res 66(2):546–553. https://doi.org/10.1161/01.res.66.2.546
Beresewicz A, Dobrzyn A, Gorski J (2002) Accumulation of specific ceramides in ischemic/reperfused heart. J Physiol Pharmacol 53(3):371–382 PMID: 12369735
Yue Y, Qin Q, Downey JM, Critz SD (2002) The relative order of mK(ATP) channels, free radicals and p38MAPK in preconditioning ‘s protective pathway in rat heart. Cardiovasc Res 55(3):681–689. https://doi.org/10.1016/s0008-6363(02)00452-2
Dalgas C, Povlsen JA, Lufgren B, Erichsen SB, Botker HE (2012) Effects of fatty acids on cardioprotection by pre-ischaemic inhibition of the malate-aspartate shuttle. Clin Exp Pharmacol Physiol 39(10):878–885.
Latipaa PM, Hiltunen JK, Peuhkurinen KJ, Hassinen IE (1983) Regulation of fatty acid oxidation in heart muscle. Effects of pyruvate and dichloroacetate. Biochim Biophys Acta 752(1):162–171. https://doi.org/10.1016/0005-2760(83)90244-8
McAllister A, Allison SP, Randle PJ (1973) Effects of dichloroacetate on the metabolism of glucose, pyruvate, acetate, 3-hydroxybutyrate and palmitate in rat diaphragm and heart muscle in vitro and on extraction of glucose, lactate, pyruvate and free fatty acids by dog heart in vivo. Biochem J 134(4):1067–1081. https://doi.org/10.1024/bj1341067
Pickavance LC, Tadayyon M, Widdowson PS, Buckingham RE, Wilding JPH (1999) Therapeutic index for rosiglitazone in dietary obese rats: separation of efficacy and haemodilution. Brit J Pharmacol 128(7):1570–1576. https://doi.org/10.1038/sj.bjp.0702932
Lochner A, Genade S, Moolman JA (2003) Ischaemic preconditioning: infarct size is more reliable endpoint than functional recovery. Basic Res Cardiol 98(5):337–346. https://doi.org/10.1007/s00395-003-0427-6
Ussher JR, Wang W, Gandhi M, Keung W et al (2012) Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 94(2):359–369. https://doi.org/10.1093/cvr/cvs/129
Salie R, Moolman JA, Lochner A (2012) The mechanism of beta-adrenergic preconditioning; role for adenosine and ROS during triggering and mediation. Basic Res Cardiol 107(5):281–303. https://doi.org/10.1007/s00395-012-0281-5
Bradford MM (1976) A rapid and sensitive method for quantification microgram quantities protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD (2002) High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency on post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 39(4):718–725. https://doi.org/10.1016/s0735-1097(01)01803-4
Ussher JR, Koves TR, Jaswal JS, Zhang L et al (2009) Insulin-stimulated cardiac glucose oxidation in increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes 58(8):1766–1775. https://doi.org/10.2335/db09-0011
Ussher JR, Lopaschuk GD (2006) Clinical implications of energetic problems in cardiovascular disease. Heart Metab 32:9–17
Saddik M, Lopaschuk GD (1991) Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266(13):8162–8170
Stanley WC, Lopaschuk GD, Hall JL, McCormack JG (1997) Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential Pharmacol Interv Cardiovasc Res 33(2):243–257. https://doi.org/10.1016/s0008-6363(96)00245-3
Fukushima A, Milner K, Gupta A, Lopaschuk GD (2015) Myocardial energy substrate metabolism in heart failure : from pathways to therapeutic targets. Curr Pharm Des 21(25):3654–3664. https://doi.org/10.2174/1381612821666150710150445
Hue L, Taegtmeyer H (2009) The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297(3):E578–E591. https://doi.org/10.1152/ajpendo.0093.2009
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90(1):207–258. https://doi.org/10.1152/physrev.00015.2009
Lopaschuk GD, Wambolt RB, Barr RL (1993) An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 264(1):135–144
Gambert S, Vergely C, Filomenko R, Moreau D, Bettaieb A, Opie LH, Rochette L (2006) Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol Cell Biochem 263(1):147–152. https://doi.org/10.1007/s11010-006-2518-9
Hausenloy DJ, Yellon DM (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12(3–4):217–234. https://doi.org/10.1007/s10741-007-9026-1
Balakumar P, Babbar L (2012) Preconditioning the hyperlipidemic myocardium: fact or fantasy? Cell Signal 24(3):589–595. https://doi.org/10.1016/j.cellsig.2011.11.003
Bolli R, Marban E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79(2):609–634. https://doi.org/10.1152/physrev.1999.79.2.609
Nadtochiy SM, Urciuoli W, Zhang J, Schafer X, Munger J, Brookes PS (2015) Metabolomic profiling of the heart during acute ischemic preconditioning reveals a role for SIRT 1in rapid cardioprotective metabolic adaptation. J Mol Cell Cardiol 88:64–72. https://doi.org/10.1016/j.yjmcc.2015.09.008
Fatima S, Hu X, Gong R-H, Huang C et al (2019) Palmitic is an intracellular signalling molecule involved in disease development. Cell Mol Life Sci 76(13):2547–2557. https://doi.org/10.1007/s00018-019-03092-7
Soltys C-LM, Buchholz L, Gandhi M, Clanachan AS, Walsh K, Dyck JR (2002) Phosphorylation of cardiac protein kinase B is regulated by palmitate. Am J Physiol Heart Circ Physiol 283(3):H1056–H1064. https://doi.org/10.1152/ajpheart.00275.2002
Sinha S et al (2004) Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279(40):41294–41301. https://doi.org/10.1074/jbc.M406514200
Aubert-Foucher E, Font B, Gautheron DC (1984) Rabbit heart mitochondrial hexokinase: solubilization and general properties. Arch Biochem Biophys 232(11):391–399. https://doi.org/10.1016/0003-9861(84)90544-x
Lawrence GM, Trayer IP (1985) The localisation of hexokinase isoenzymes in red and white skeletal muscles of the rat. Histochem J 17(3):353–371. https://doi.org/10.1007/bf01004597
Ritov VB, Kellek DE (2001) Hexokinase isozyme distribution in human skeletal muscle. Diabetes 50(6):1256–1262. https://doi.org/10.2337/diabetes.50.6.1253
Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localisation and metabolic function. J Exp Biol 206(12):2049–2057. https://doi.org/10.1242/jeb.00241
Southworth R, Davey KAB, Warley A, Garlick PB (2007) A reevaluation of the roles of Hexokinase I and II in the heart. Am J Physiol Heart Circ Physiol 292(1):H378–H386. https://doi.org/10.1152/ajpheart.00664.2006
Smeele KM, Southworth R, Wu R, Xie C, Nederlof R et al (2011) Disruption of hexokinase II-mitochondrial binding blocks ischemic preconditioning and causes rapid cardiac fibrosis. Circ Res 108(10):1165–1169. https://doi.org/10.1161/CIRCRESAHA.111.244962
Nederlof R, Eerbeek O, Hollmann MWW, Siuthworth R, Zuurbier CJ (2014) Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in the heart. Br J Pharmacol 171(8):2067–2079. https://doi.org/10.1111/bph.12363
Bromage DI, Pickard JM, Rossello X et al (2017) Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: a systematic review and meta-analysis. Cardiovasc Res 113(3):288–297. https://doi.org/10.1093/cvr/cvw219
Rossello X, He Z, Yellon DM (2018) Myocardial infarct size reduction provided by local and remote ischaemic preconditioning: reference values from the Hatter Cardiovascular Institute. Cardiovasc Drugs Ther 32(2):127–133. https://doi.org/10.1007/s10557-018-6788-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lochner, A., Genade, S., Genis, A. et al. Long-chain free fatty acids inhibit ischaemic preconditioning of the isolated rat heart. Mol Cell Biochem 473, 111–132 (2020). https://doi.org/10.1007/s11010-020-03812-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03812-9