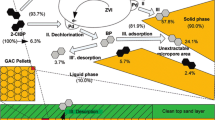
Abstract
Capping represents an efficient and well-established remediation practice to contain pollutants in sediments. Coupling capping amendments with biodegradation is an emerging technology with great potential to promote simultaneous sequestration and oxidation of contaminants in situ. Capping materials alter the native sediment environment and affect the biodegradation potential of benthic microbial communities. The placement of materials specifically influences (i) porewater pH and composition, (ii) nutrient fluxes, (iii) electron accepting processes, (iv) bioavailability of contaminants, and (v) biofilm formation. This review summarizes current literature documenting the impact of these alterations on microbial ecology and biodegradation activity, describes recent advances in bioactive sediment caps, and identifies areas where additional research is warranted.

Similar content being viewed by others
Data Availability
(Data transparency): Not applicable
References
Kan AT, Fu G, Tomson MB. Adsorption/desorption hysteresis in organic pollutant and soil/sediment interaction. Environmental Science & Technology. 1994;28(5):859–67.
Cornelissen G, van Noort PC, Govers HA. Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: sediment extraction with Tenax® and effects of contact time and solute hydrophobicity. Environmental Toxicology and Chemistry: An International Journal. 1997;16(7):1351–7.
Cornelissen G, van Noort PC, Govers HA. Mechanism of slow desorption of organic compounds from sediments: a study using model sorbents. Environmental Science & Technology. 1998;32(20):3124–31.
Reible DD, editor. Processes, assessment and remediation of contaminated sediments. New York: Springer; 2014.
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS. Role of assisted natural remediation in environmental cleanup. Geoderma. 2004;122(2–4):121–42.
Lofrano G, Libralato G, Minetto D, De Gisi S, Todaro F, Conte B, et al. In situ remediation of contaminated marinesediment: an overview. Environ Sci Pollut Res. 2017;24(6):5189–206.
Wang XQ, Thibodeaux LJ, Valsaraj KT, Reible DD. Efficiency of capping contaminated bed sediments in situ. 1. Laboratory-scale experiments on diffusion-adsorption in the capping layer. Environmental Science & Technology. 1991;25(9):1578–84.
Thoma GJ, Reible DD, Valsaraj KT, Thibodeaux LJ. Efficiency of capping contaminated sediments in situ. 2. Mathematics of diffusion-adsorption in the capping layer. Environmental Science & Technology. 1993;27(12):2412–9.
Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AI. The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol. 2016;14(4):251–63.
Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater. 2009;169(1–3):1–5.
Zhang C, Zhu MY, Zeng GM, Yu ZG, Cui F, Yang ZZ, et al. Active capping technology: a new environmental remediation of contaminated sediment. Environ Sci Pollut Res. 2016;23(5):4370–86.
Islam MS, Zhang Y, McPhedran KN, Liu Y, El-Din MG. Mechanistic investigation of industrial wastewater naphthenic acids removal using granular activated carbon (GAC) biofilm based processes. Sci Total Environ. 2016;541:238–46.
Kalmykova Y, Moona N, Strömvall AM, Björklund K. Sorption and degradation of petroleum hydrocarbons, polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachate using sand, activated carbon and peat filters. Water Res. 2014;56:246–57.
Piai L, Blokland M, van der Wal A, Langenhoff A. Biodegradation and adsorption of micropollutants by biological activated carbon from a drinking water production plant. J Hazard Mater. 2020;7:122028.
Simsek H, Kasi M, Ohm JB, Blonigen M, Khan E. Bioavailable and biodegradable dissolved organic nitrogen in activated sludge and trickling filter wastewater treatment plants water research. 2013;47(9):3201–10.
Majcher EH, Lorah MM, Phelan DJ, McGinty AL. Design and performance of an enhanced bioremediation pilot test in a tidal wetland seep. Aberdeen Proving Ground, Maryland. US Department of the Interior, US Geological Survey: West Branch Canal Creek; 2009.
Carroll SM, Barrett JK, Haswell WJ, Fisher WR, Okin MB. Field-scale pilot test of a reactive core mat to address coal tar seepage. Presentation at the Battelle Contaminated Sediments Conference, 2011.
Carroll SM. Haswell WJ. Design of a reactive cap remedy for soft, NAPL-impacted sediments. Presentation at the Sediment Management Work Group, May 21, 2014.
Smith KL. Anaerobic degradation of polycyclic aromatic hydrocarbons at a creosote-contaminated superfund site and the significance of increased methane production in an organophilic clay sediment cap. 2010.
Carroll SM, Peacock AD, Zimbron J, Alepidis KN, Clock JA. Demonstrating contaminant degradation at an MGP site with metabolic gas flux and radio carbon dating. Remediat J. 2017;27(2):51–64.
Smith AM. Microbiological activity and organic pollutant fate and transport in sediments and sediment caps. 2011 (Doctoral dissertation).
Smith AM, Kirisits MJ, Reible DD. Assessment of potential anaerobic biotransformation of organic pollutants in sediment caps. New Biotechnol. 2012;30(1):80–7.
Vlassopoulos D, Russell K, Larosa P, Brown R, Mohan R, Glaza E, et al. Evaluation, design, and construction of amended reactive caps to restore Onondaga Lake, Syracuse, New York. USA Journal of Marine Environmental Engineering. 2017;1:10(1).
Go J, Lampert DJ, Stegemann JA, Reible DD. Predicting contaminant fate and transport in sediment caps: mathematical modelling approaches. Appl Geochem. 2009;24(7):1347–53.
Shen X, Lampert D, Ogle S, Reible D. A software tool for simulating contaminant transport and remedial effectiveness in sediment environments. Environ Model Softw. 2018;109:104–13.
Bertics VJ, Ziebis W. Biodiversity of benthic microbial communities in bioturbated coastal sediments is controlled by geochemical microniches. The ISME Journal. 2009;3(11):1269–85.
Shimabuku KK, Kennedy AM, Mulhern RE, Summers RS. Evaluating activated carbon adsorption of dissolved organic matter and micropollutants using fluorescence spectroscopy. Environmental Science & Technology. 2017;51(5):2676–84.
Summers RS, Kim SM, Shimabuku K, Chae SH, Corwin CJ. Granular activated carbon adsorption of MIB in the presence of dissolved organic matter. Water Res. 2013;47(10):3507–13.
Haftka JJ, Parsons JR, Govers HA, Ortega-Calvo JJ. Enhanced kinetics of solid-phase microextraction and biodegradation of polycyclic aromatic hydrocarbons in the presence of dissolved organic matter. Environmental Toxicology and Chemistry: An International Journal. 2008;27(7):1526–32.
Smith KE, Thullner M, Wick LY, Harms H. Sorption to humic acids enhances polycyclic aromatic hydrocarbon biodegradation. Environmental Science & Technology. 2009;43(19):7205–11.
Alvisi F, Cibic T, Fazi S, Bongiorni L, Relitti F, Del Negro P. Role of depositional dynamics and riverine input in shaping microbial benthic community structure of Po prodelta system (NW Adriatic, Italy). Estuarine, Coastal and Shelf Science. 2019;227:106305.
Huang T, Xu J, Cai D. Efficiency of active barriers attaching biofilm as sediment capping to eliminate the internal nitrogen in eutrophic lake and canal. J Environ Sci. 2011;23(5):738–43.
Wingenfelder U, Hansen C, Furrer G, Schulin R. Removal of heavy metals from mine waters by natural zeolites. Environmental Science & Technology. 2005;39(12):4606–13.
Xiong C, Wang D, Tam NF, Dai Y, Zhang X, Tang X, et al. Enhancement of active thin-layer capping with natural zeolite to simultaneously inhibit nutrient and heavy metal release from sediments. Ecol Eng. 2018;119:64–72.
Huang T, Zhou Z, Xu J, Dong Y, Wang G. Biozeolite capping for reducing nitrogen load of the ancient canal in Yangzhou City. Water Sci Technol. 2012;66(2):336–44.
Huang T, Zhou Z, Su J, Dong Y, Wang G. Nitrogen reduction in a eutrophic river canal using bioactive multilayer capping (BMC) with biozeolite and sand. J Soils Sediments. 2013;13(7):1309–17.
Sterner RW, Elser JJ. Ecological stoichiometry: the biology of elements from molecules to the biosphere: Princeton University Press; 2002.
Wolicka D, Suszek A, Borkowski A, Bielecka A. Application of aerobic microorganisms in bioremediation in situ of soil contaminated by petroleum products. Bioresour Technol. 2009;100(13):3221–7.
Orland C, Emilson EJ, Basiliko N, Mykytczuk NC, Gunn JM, Tanentzap AJ. Microbiome functioning depends on individual and interactive effects of the environment and community structure. The ISME Journal. 2019;13(1):1–11.
Nikolopoulou M, Kalogerakis N. Enhanced bioremediation of crude oil utilizing lipophilic fertilizers combined with biosurfactants and molasses. Mar Pollut Bull. 2008;56(11):1855–61.
Swannell RP, Lee K, McDonagh M. Field evaluations of marine oil spill bioremediation. Microbiol Mol Biol Rev. 1996;60(2):342–65.
Bonaglia S, Rämö R, Marzocchi U, Le Bouille L, Leermakers M, Nascimento FJ, et al. Capping with activated carbon reduces nutrient fluxes, denitrification and meiofauna in contaminated sediments. Water Res. 2019;148:515–25.
Mohan D, Pittman CU Jr. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J Hazard Mater. 2006;137(2):762–811.
Beaulne JS, Mishra SR, Suar M, Panda AN, Rastogi G, Pattnaik AK, et al. Spatial analysis of bacteria in brackish lake sediment. International Journal of Sediment Research. 2020.
Liu S, Ren H, Shen L, Lou L, Tian G, Zheng P, et al. pH levels drive bacterial community structure in sediments of the Qiantang River as determined by 454 pyrosequencing. Front Microbiol. 2015;6:285.
Silburn B, Kröger S, Parker ER, Sivyer DB, Hicks N, Powell CF, et al. Benthic pH gradients across a range of shelf sea sediment types linked to sediment characteristics and seasonal variability. Biogeochemistry. 2017;135(1–2):69–88.
Wu K, Zhao W, Wang Q, Yang X, Zhu L, Shen J, et al. The relative abundance of benthic bacterial Phyla along a water-depth gradient in a plateau Lake: physical, chemical, and biotic drivers. Front Microbiol. 2019;10.
Glöckner FO, Fuchs BM, Amann R. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl Environ Microbiol. 1999;65(8):3721–6.
Sekar R, Fuchs BM, Amann R, Pernthaler J. Flow sorting of marine bacterioplankton after fluorescence in situ hybridization. Appl Environ Microbiol. 2004;70(10):6210–9.
Koelmans AA, Jonker MT. Effects of black carbon on bioturbation-induced benthic fluxes of polychlorinated biphenyls. Chemosphere. 2011;84(8):1150–7.
Millward RN, Bridges TS, Ghosh U, Zimmerman JR, Luthy RG. Addition of activated carbon to sediments to reduce PCB bioaccumulation by a polychaete (Neanthes arenaceodentata) and an amphipod (Leptocheirus plumulosus). Environmental Science & Technology. 2005;39(8):2880–7.
Nybom I, Werner D, Leppänen MT, Siavalas G, Christanis K, Karapanagioti HK, et al. Responses of Lumbriculus variegatus to activated carbon amendments in uncontaminated sediments. Environmental Science & Technology. 2012;46(23):12895–903.
Tomaszewski JE, McLeod PB, Luthy RG. Measuring and modeling reduction of DDT availability to the water column and mussels following activated carbon amendment of contaminated sediment. Water Res. 2008;42(16):4348–56.
Cornelissen G, Elmquist Kruså M, Breedveld GD, Eek E, Oen AM, Arp HP, et al. Remediation of contaminated marine sediment using thin-layer capping with activated carbon—a field experiment in Trondheim Harbor, Norway. Environmental Science & Technology. 2011;45(14):6110–6.
Gomez-Eyles JL, Yupanqui C, Beckingham B, Riedel G, Gilmour C, Ghosh U. Evaluation of biochars and activated carbons for in situ remediation of sediments impacted with organics, mercury, and methylmercury. Environmental Science & Technology. 2013;47(23):13721–9.
Kupryianchyk D, Peeters ET, Rakowska MI, Reichman EP, Grotenhuis JT, Koelmans AA. Long-term recovery of benthic communities in sediments amended with activated carbon. Environmental Science & Technology. 2012;46(19):10735–42.
Nybom I, Waissi-Leinonen G, Mäenpää K, Leppänen MT, Kukkonen JV, Werner D, et al. Effects of activated carbon ageing in three PCB contaminated sediments: sorption efficiency and secondary effects on Lumbriculus variegatus. Water Res. 2015;85:413–21.
Schallenberg M, Kalff J. The ecology of sediment bacteria in lakes and comparisons with other aquatic ecosystems. Ecology. 1993;74(3):919–34.
Quero GM, Cassin D, Botter M, Perini L, Luna GM. Patterns of benthic bacterial diversity in coastal areas contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Front Microbiol. 2015;6:1053.
Sun MY, Dafforn KA, Johnston EL, Brown MV. Core sediment bacteria drive community response to anthropogenic contamination over multiple environmental gradients. Environ Microbiol. 2013;15(9):2517–31.
Morel FM, Hering JG. Principles and applications of aquatic chemistry: John Wiley & Sons; 1993.
Ruiz-González C, Niño-García JP, del Giorgio PA. Terrestrial origin of bacterial communities in complex boreal freshwater networks. Ecol Lett. 2015;18(11):1198–206.
Bernhard JM, Reimers CE. Benthic foraminiferal population fluctuations related to anoxia: Santa Barbara Basin. Biogeochemistry. 1991;15(2):127–49.
Himmelheber DW, Thomas SH, Löffler FE, Taillefert M, Hughes JB. Microbial colonization of an in situ sediment cap and correlation to stratified redox zones. Environmental Science & Technology. 2009;43(1):66–74.
Fahrenfeld N, Pruden A, Widdowson M. Kinetic and microbial community analysis of methyl ethyl ketone biodegradation in aquifer sediments. Biodegradation. 2017;28(1):27–36.
Yang S, Gou Y, Song Y, Li P. Enhanced anoxic biodegradation of polycyclic aromatic hydrocarbons (PAHs) in a highly contaminated aged soil using nitrate and soil microbes. Environ Earth Sci. 2018;77(12):432.
Yang X, Li E, Liu F, Xu M. Interactions of PAH-degradation and nitrate−/sulfate-reducing assemblages in anaerobic sediment microbial community. J Hazard Mater. 2020;10:122068.
Meckenstock RU, Boll M, Mouttaki H, Koelschbach JS, Tarouco PC, Weyrauch P, et al. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J Mol Microbiol Biotechnol. 2016;26(1–3):92–118.
Johnson NW, Reible DD, Katz LE. Biogeochemical changes and mercury methylation beneath an in-situ sediment cap. Environmental Science & Technology. 2010;44(19):7280–6.
Sowers KR, May HD. In situ treatment of PCBs by anaerobic microbial dechlorination in aquatic sediment: are we there yet? Curr Opin Biotechnol. 2013;24(3):482–8.
Seeger M, Hernández M, Méndez V, Ponce B, Córdova M, González M. Bacterial degradation and bioremediation of chlorinated herbicides and biphenyls. J Soil Sci Plant Nutr. 2010;10(3):320–32.
Ghosh U, Weber AS, Jensen JN, Smith JR. Relationship between PCB desorption equilibrium, kinetics, and availability during land biotreatment. Environmental Science & Technology. 2000;34(12):2542–8.
Liu X, Sokol RC, Kwon OS, Bethoney CM, Rhee GY. An investigation of factors limiting the reductive dechlorination of polychlorinated biphenyls. Environmental Toxicology and Chemistry: An International Journal. 1996;15(10):1738–44.
Hale SE, Meynet P, Davenport RJ, Jones DM, Werner D. Changes in polycyclic aromatic hydrocarbon availability in River Tyne sediment following bioremediation treatments or activated carbon amendment. Water Res. 2010;44(15):4529–36.
Yan F, Reible D. Electro-bioremediation of contaminated sediment by electrode enhanced capping. J Environ Manag. 2015;155:154–61.
Ueki T, Nevin KP, Rotaru AE, Wang LY, Ward JE, Woodard TL, et al. Geobacter strains expressing poorly conductive Pili reveal constraints on direct interspecies electron transfer mechanisms. MBio. 2018;9(4):e01273–18.
Lovley DR. Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ Sci. 2011;4(12):4896–906.
Bonaglia S, Broman E, Brindefalk B, Hedlund E, Hjorth T, Rolff C, et al. Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere. 2020;24:126023.
Min D, Cheng L, Zhang F, Huang XN, Li DB, Liu DF, et al. Enhancing extracellular electron transfer of Shewanella oneidensis MR-1 through coupling improved flavin synthesis and metal-reducing conduit for pollutant degradation. Environmental Science & Technology. 2017;51(9):5082–9.
Liu F, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. Promoting direct interspecies electron transfer with activated carbon. Energy Environ Sci. 2012;5(10):8982–9.
Dubé CD, Guiot SR. Direct interspecies electron transfer in anaerobic digestion: a review. In: Biogas science and technology. Cham: Springer; 2015. p. 101–15.
Martins G, Salvador AF, Pereira L, Alves MM. Methane production and conductive materials: a critical review. Environmental Science & Technology. 2018;52(18):10241–53.
Lovley DR. Happy together: microbial communities that hook up to swap electrons. The ISME Journal. 2017;11(2):327–36.
McGlynn SE, Chadwick GL, Kempes CP, Orphan VJ. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature. 2015;526(7574):531–5.
Sun T, Levin BD, Guzman JJ, Enders A, Muller DA, Angenent LT, et al. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat Commun. 2017;8:14873.
Poot A, Jonker MT, Gillissen F, Koelmans AA. Explaining PAH desorption from sediments using Rock Eval analysis. Environ Pollut. 2014;193:247–53.
Niehus NC, Brockmeyer B, Witt G. Bioavailability and distribution of PAHs and PCBs in the sediment pore water of the German Bight and Wadden Sea. Mar Pollut Bull. 2019;138:421–7.
Rakowska MI, Smit MP, Kupryianchyk D, Qin J, Koelmans AA, Rijnaarts HH, et al. Turbulent mixing accelerates PAH desorption due to fragmentation of sediment particle aggregates. J Soils Sediments. 2017;17(1):277–85.
Ren X, Zeng G, Tang L, Wang J, Wan J, Liu Y, et al. Sorption, transport and biodegradation–an insight into bioavailability of persistent organic pollutants in soil. Sci Total Environ. 2018;610:1154–63.
Gidley PT, Kwon S, Yakirevich A, Magar VS, Ghosh U. Advection dominated transport of polycyclic aromatic hydrocarbons in amended sediment caps. Environmental Science & Technology. 2012;46(9):5032–9.
Amstaetter K, Eek E, Cornelissen G. Sorption of PAHs and PCBs to activated carbon: coal versus biomass-based quality. Chemosphere. 2012;87(5):573–8.
Carvalho MF, Duque AF, Gonçalves IC, Castro PM. Adsorption of fluorobenzene onto granular activated carbon: isotherm and bioavailability studies. Bioresour Technol. 2007;98(18):3424–30.
Jensen B, Kuznetsova T, Kvamme B, Oterhals Å. Molecular dynamics study of selective adsorption of PCB on activated carbon. Fluid Phase Equilib. 2011;307(1):58–65.
Abromaitis V, Racys V, Van Der Marel P, Meulepas RJ. Biodegradation of persistent organics can overcome adsorption–desorption hysteresis in biological activated carbon systems. Chemosphere. 2016;149:183–9.
El Gamal M, Mousa HA, El-Naas MH, Zacharia R, Judd S. Bio-regeneration of activated carbon: a comprehensive review. Sep Purif Technol. 2018;197:345–59.
Choi H, Agarwal S, Al-Abed SR. Adsorption and simultaneous dechlorination of PCBs on GAC/Fe/Pd: mechanistic aspects and reactive capping barrier concept. Environmental science & Technology. 2009;43(2):488–93.
Igun OT, Meynet P, Davenport RJ, Werner D. Impacts of activated carbon amendments, added from the start or after five months, on the microbiology and outcomes of crude oil bioremediation in soil. Int Biodeterior Biodegradation. 2019;142:1–10.
Ma X, Li N, Jiang J, Xu Q, Li H, Wang L, et al. Adsorption–synergic biodegradation of high-concentrated phenolic water by Pseudomonas putida immobilized on activated carbon fiber. Journal of Environmental Chemical Engineering. 2013;1(3):466–72.
Ma B, Arnold WA, Hozalski RM. The relative roles of sorption and biodegradation in the removal of contaminants of emerging concern (CECs) in GAC-sand biofilters. Water Res. 2018;146:67–76.
Cheng G, Sun M, Ge X, Xu X, Lin Q, Lou L. Exploration of biodegradation mechanisms of black carbon-bound nonylphenol in black carbon-amended sediment. Environ Pollut. 2017;231:752–60.
Islam MS, Zhang Y, McPhedran KN, Liu Y, El-Din MG. Granular activated carbon for simultaneous adsorption and biodegradation of toxic oil sands process-affected water organic compounds. J Environ Manag. 2015;152:49–57.
Marchal G, Smith KE, Rein A, Winding A, Trapp S, Karlson UG. Comparing the desorption and biodegradation of low concentrations of phenanthrene sorbed to activated carbon, biochar and compost. Chemosphere. 2013;90(6):1767–78.
Shi J, Han Y, Xu C, Han H. Enhanced biodegradation of coal gasification wastewater with anaerobic biofilm on polyurethane (PU), powdered activated carbon (PAC), and biochar. Bioresour Technol. 2019;289:121487.
Velten S, Boller M, Köster O, Helbing J, Weilenmann HU, Hammes F. Development of biomass in a drinking water granular active carbon (GAC) filter. Water Res. 2011;45(19):6347–54.
Hejazi F, Ghoreyshi AA, Rahimnejad M. Simultaneous phenol removal and electricity generation using a hybrid granular activated carbon adsorption-biodegradation process in a batch recycled tubular microbial fuel cell. Biomass Bioenergy. 2019;129:105336.
Needham TP, Payne RB, Sowers KR, Ghosh U. Kinetics of PCB microbial dechlorination explained by freely dissolved concentration in sediment microcosms. Environmental Science & Technology. 2019;53(13):7432–41.
Liang Y, Zhang X, Dai D, Li G. Porous biocarrier-enhanced biodegradation of crude oil contaminated soil. Int Biodeterior Biodegradation. 2009;63(1):80–7.
Shukla SK, Mangwani N, Rao TS. Bioremediation approaches for persistent organic pollutants using microbial biofilms. Microbial Biofilms in Bioremediation and Wastewater Treatment. 2019;18:179.
Edwards SJ, Kjellerup BV. Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl Microbiol Biotechnol. 2013;97(23):9909–21.
Yan S, Wu G. Reorganization of gene network for degradation of polycyclic aromatic hydrocarbons (PAHs) in Pseudomonas aeruginosa PAO1 under several conditions. J Appl Genet. 2017;58(4):545–63.
Mangwani N, Kumari S, Das S. Bacterial biofilms and quorum sensing: fidelity in bioremediation technology. Biotechnol Genet Eng Rev. 2016;32(1–2):43–73.
Martirani-Von Abercron SM, Marín P, Solsona-Ferraz M, Castañeda-Cataña MA, Marqués S. Naphthalene biodegradation under oxygen-limiting conditions: community dynamics and the relevance of biofilm-forming capacity. Microb Biotechnol. 2017 Nov;10(6):1781–96.
Freidman BL, Gras SL, Snape I, Stevens GW, Mumford KA. A bio-reactive barrier sequence for petroleum hydrocarbon capture and degradation in low nutrient environments. Int Biodeterior Biodegradation. 2017;116:26–37.
Singh R, Paul D, Jain RK. Biofilms: implications in bioremediation. Trends Microbiol. 2006;14(9):389–97.
Luthy RG, Ghosh U, Inventors; Leland Stanford Junior University, assignee. In situ stabilization of persistent hydrophobic organic contaminants in sediments using coal-and wood-derived carbon sorbents. United States patent US 7,101,115. 2006.
Abbate C, Arena M, Baglieri A, Gennari M. Effects of organoclays on soil eubacterial community assessed by molecular approaches. J Hazard Mater. 2009;168(1):466–72.
Biswas B, Juhasz AL, Mahmudur Rahman M, Naidu R. Modified clays alter diversity and respiration profile of microorganisms in long-term hydrocarbon and metal co-contaminated soil. Microb Biotechnol. 2020;13(2):522–34.
Ugochukwu UC, Manning DA, Fialips CI. Microbial degradation of crude oil hydrocarbons on organoclay minerals. J Environ Manag. 2014 Nov 1;144:197–202.
Ugochukwu UC, Fialips CI. Removal of crude oil polycyclic aromatic hydrocarbons via organoclay-microbe-oil interactions. Chemosphere. 2017;174:28–38.
Chan S, Pullerits K, Riechelmann J, Persson KM, Rådström P, Paul CJ. Monitoring biofilm function in new and matured full-scale slow sand filters using flow cytometric histogram image comparison (CHIC). Water Res. 2018;138:27–36.
Lautenschlager K, Hwang C, Ling F, Liu WT, Boon N, Köster O, et al. Abundance and composition of indigenous bacterial communities in a multi-step biofiltration-based drinking water treatment plant. Water Res. 2014;62:40–52.
Lyautey E, Jackson CR, Cayrou J, Rols JL, Garabétian F. Bacterial community succession in natural river biofilm assemblages. Microb Ecol. 2005;50(4):589–601.
Kjelleberg S, Givskov M. Biofilm mode of life. Horizon Bioscience; 2007.
Costerton J. The biofilm primer (Springer series on biofilms). Berlin: Springer; 2007a.
Macedo AJ, Kuhlicke U, Neu TR, Timmis KN, Abraham WR. Three stages of a biofilm community developing at the liquid-liquid interface between polychlorinated biphenyls and water. Appl Environ Microbiol. 2005;71(11):7301–9.
Fang H, Fazeli M, Cheng W, Huang L, Hu H. Biostabilization and transport of cohesive sediment deposits in the Three Gorges Reservoir. PloS One. 2015;10(11).
Mercier A, Wille G, Michel C, Harris-Hellal J, Amalric L, Morlay C, et al. Biofilm formation vs. PCB adsorption on granular activated carbon in PCB-contaminated aquatic sediment. J Soils Sediments. 2013;13(4):793–800.
Jiao S, Chen W, Wang E, Wang J, Liu Z, Li Y, et al. Microbial succession in response to pollutants in batch-enrichment culture. Sci Rep. 2016;6:21791.
Mercier A, Joulian C, Michel C, Auger P, Coulon S, Amalric L, et al. Evaluation of three activated carbons for combined adsorption and biodegradation of PCBs in aquatic sediment. Water Res. 2014;59:304–15.
Mercier A, Michel C, Joulian C, Touzé S, Amalric L, Bataillard P, et al. Decrease of the level of extractable polychlorinated biphenyls in soil microcosms: influence of granular activated carbon and inoculation by natural microbial consortia. Int Biodeterior Biodegradation. 2015;105:127–36.
Meynet P, Hale SE, Davenport RJ, Cornelissen G, Breedveld GD, Werner D. Effect of activated carbon amendment on bacterial community structure and functions in a PAH impacted urban soil. Environmental Science & Technology. 2012;46(9):5057–66.
Oh WD, Lim PE, Seng CE, Sujari AN. Kinetic modeling of bioregeneration of chlorophenol-loaded granular activated carbon in simultaneous adsorption and biodegradation processes. Bioresour Technol. 2012;114:179–87.
Hyun S, Jafvert CT, Lee LS, Rao PS. Laboratory studies to characterize the efficacy of sand capping a coal tar-contaminated sediment. Chemosphere. 2006;63(10):1621–31.
Murphy P, Marquette A, Reible D, Lowry GV. Predicting the performance of activated carbon-, coke-, and soil-amended thin layer sediment caps. J Environ Eng. 2006;132(7):787–94.
Himmelheber DW, Pennell KD, Hughes JB. Evaluation of a laboratory-scale bioreactive in situ sediment cap for the treatment of organic contaminants. Water Res. 2011;45(17):5365–74.
Kim YS, Nyberg LM, Jenkinson B, Jafvert CT. PAH concentration gradients and fluxes through sand cap test cells installed in situ over river sediments containing coal tar. Environmental Science: Processes & Impacts. 2013;15(8):1601–12.
Wang Q, Li Y, Wang C, Wu Y, Wang P. Development of a novel multi-functional active membrane capping barrier for the remediation of nitrobenzene-contaminated sediment. J Hazard Mater. 2014;276:415–21.
Ali O, Namane A, Hellal A. Use and recycling of Ca-alginate biocatalyst for removal of phenol from wastewater. J Ind Eng Chem. 2013;19(4):1384–90.
Al-Zuhair S, El-Naas M. Immobilization of Pseudomonas putida in PVA gel particles for the biodegradation of phenol at high concentrations. Biochem Eng J. 2011;56(1–2):46–50.
Chen Y, Yu B, Lin J, Naidu R, Chen Z. Simultaneous adsorption and biodegradation (SAB) of diesel oil using immobilized Acinetobacter venetianus on porous material. Chem Eng J. 2016;289:463–70.
Deng F, Liao C, Yang C, Guo C, Dang Z. Enhanced biodegradation of pyrene by immobilized bacteria on modified biomass materials. Int Biodeterior Biodegradation. 2016;110:46–52.
Gong B, Wu P, Huang Z, Li Y, Dang Z, Ruan B, et al. Enhanced degradation of phenol by Sphingomonas sp. GY2B with resistance towards suboptimal environment through adsorption on kaolinite. Chemosphere. 2016;148:388–94.
Gong B, Wu P, Ruan B, Zhang Y, Lai X, Yu L, et al. Differential regulation of phenanthrene biodegradation process by kaolinite and quartz and the underlying mechanism. J Hazard Mater. 2018;349:51–9.
Jin X, Tian W, Liu Q, Qiao K, Zhao J, Gong X. Biodegradation of the benzo (a) pyrene-contaminated sediment of the Jiaozhou Bay wetland using Pseudomonas sp. immobilization. Mar Pollut Bull. 2017;117(1–2):283–90.
Kureel MK, Geed SR, Giri BS, Rai BN, Singh RS. Biodegradation and kinetic study of benzene in bioreactor packed with PUF and alginate beads and immobilized with Bacillus sp. M3. Bioresource Technology. 2017;242:92–100.
Lin Q, Donghui W, Jianlong W. Biodegradation of pyridine by Paracoccus sp. KT-5 immobilized on bamboo-based activated carbon. Bioresour Technol. 2010;101(14):5229–34.
Omarova M, Swientoniewski LT, Tsengam IK, Panchal A, Yu T, Blake DA, et al. Engineered clays as sustainable oil dispersants in the presence of model hydrocarbon degrading bacteria: the role of bacterial sequestration and biofilm formation. ACS Sustain Chem Eng. 2018;6(11):14143–53.
Payne RB, Ghosh U, May HD, Marshall CW, Sowers KR. Mesocosm studies on the efficacy of bioamended activated carbon for treating PCB-impacted sediment. Environmental Science & Technology. 2017;51(18):10691–9.
Payne RB, Ghosh U, May HD, Marshall CW, Sowers KR. A pilot-scale field study: in situ treatment of PCB-impacted sediments with bioamended activated carbon. Environmental Science & Technology. 2019;53(5):2626–34.
Singh N, Balomajumder C. Simultaneous biosorption and bioaccumulation of phenol and cyanide using coconut shell activated carbon immobilized Pseudomonas putida (MTCC 1194). Journal of Environmental Chemical Engineering. 2016;4(2):1604–14.
Toh RH, Lim PE, Seng CE, Adnan R. Immobilized acclimated biomass-powdered activated carbon for the bioregeneration of granular activated carbon loaded with phenol and o-cresol. Bioresour Technol. 2013;143:265–74.
Xiong B, Zhang Y, Hou Y, Arp HP, Reid BJ, Cai C. Enhanced biodegradation of PAHs in historically contaminated soil by M gilvum inoculated biochar. Chemosphere. 2017;182:316–24.
Zapata Acosta K, Carrasco-Marin F, Cortés FB, Franco CA, Lopera SH, Rojano BA. Immobilization of P. stutzeri on activated carbons for degradation of hydrocarbons from oil-in-saltwater emulsions. Nanomaterials. 2019;9(4):500.
Chung TP, Tseng HY, Juang RS. Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem. 2003;38(10):1497–507.
Zheng Y, Chen D, Li N, Xu Q, Li H, He J, et al. Highly efficient simultaneous adsorption and biodegradation of a highly concentrated anionic dye by a high-surface-area carbon-based biocomposite. Chemosphere. 2017;179:139–47.
Chun CL, Payne RB, Sowers KR, May HD. Electrical stimulation of microbial PCB degradation in sediment. Water Res. 2013;47(1):141–52.
Di Gregorio S, Azaizeh H, Lorenzi R. Biostimulation of the autochthonous microbial community for the depletion of polychlorinated biphenyls (PCBs) in contaminated sediments. Environ Sci Pollut Res. 2013;20(6):3989–99.
Sun M, Yan F, Zhang R, Reible DD, Lowry GV, Gregory KB. Redox control and hydrogen production in sediment caps using carbon cloth electrodes. Environmental Science & Technology. 2010;44(21):8209–15.
Yan F, Reible DD. PAH degradation and redox control in an electrode enhanced sediment cap. J Chem Technol Biotechnol. 2012;87(9):1222–8.
Starr RC, Cherry JA. In situ remediation of contaminated ground water: the funnel-and-gate system. Groundwater. 1994;32(3):465–76.
Yan F, Reible DD. Modeling of funnel and gate Systems for Remediation of contaminated sediment. In: Fluid dynamics in physics, engineering and environmental applications. Berlin, Heidelberg: Springer; 2013. p. 391–400.
Code Availability
(Software application or custom code): Not applicable
Funding
This work was supported by a Dissertation Completion Fellowship awarded to Giovanna Pagnozzi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sediment Pollution
Rights and permissions
About this article
Cite this article
Pagnozzi, G., Carroll, S., Reible, D.D. et al. Biological Natural Attenuation and Contaminant Oxidation in Sediment Caps: Recent Advances and Future Opportunities. Curr Pollution Rep 6, 281–294 (2020). https://doi.org/10.1007/s40726-020-00153-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-020-00153-5