Abstract
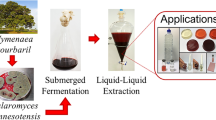
In this study, bioproduction and application of a microbial pigment from Chryseobacterium and Deinococcus species screened from soil were investigated. The pigments extracted from both cell cultures were identified as a flexirubin (FL)- and deinoxanthin-type (DX). The isolated pigments showed a mass value of 618.48 and 584.41 m/z by LC/MS, respectively. Flask production of the crude FL pigment in complex medium yielded 0.443 ± 0.047 g/L, while crude DX production was less than 20 mg/L. Similar to previously identified FL and DX pigments, both have unsaturated long-chain alkyl-substituted phenol and cyclo-hexanone units and showed excellent radical scavenging capacities of 0.6 and 3.2 mg/mL IC50 values, respectively. In order to utilize the pigments for functional painting materials, both pigments were mixed with casein paint to prepare FL bio-paint and DX bio-paint, respectively. Interestingly, yellow-colored FL bio-paint and red-colored DX bio-paint showed an outstanding coloration and coating performance onto glass plate. Furthermore, it was confirmed that the produced DX bio-paint had a unique crystal structure when analyzed by SEM.
Similar content being viewed by others
References
Tuli, H. S., P. Chaudhary, V. Beniwal, and A. K. Sharma (2015) Microbial pigments as natural color sources: current trends and future perspectives J. Food Sci. Technol. 52: 4669–4678.
Baez, L. A., J. Santos, P. Ramirez, L. A. Trujillo-Cayado, and J. Munoz (2019) Development of emulgels formulated with sweet fennel oil and rhamsan gum, a biological macromolecule produced by Sphingomonas. Int. J. Biol. Macromol. 129: 326–332.
Chandika, P., S. C. Ko, and W. K. Jung (2015) Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration Int. J. Biol. Macromol. 11: 24–35.
Hu, Y. L., J. Y. Luo, H. Z. Zhao, S. S. Zhang, S. H. Yang, and M. H. Yang (2016) Application of natural plant pigment in hair dyes Zhongguo Zhong Yao Za Zhi. 41: 3226–3231.
Masuelli, L., F. Pantanella, G. La Regina, M. Benvenuto, M. Fantini, R. Mattera, E. Di Stefano, M. Mattei, R. Silvestri, S. Schippa, V. Manzari, A. Modesti, and R. Bei (2016) Violacein, an indole-derived purple-colored natural pigment produced by Janthinobacterium lividum, inhibits the growth of head and neck carcinoma cell lines both in vitro and in vivo. Tumour. Biol. 37: 3705–3717.
Irimia-Vladu, M., E. D. Glowacki, P. A. Troshin, G. Schwabegger, L. Leonat, D. K. Susarova, O. Krystal, M. Ullah, Y. Kanbur, M. A. Bodea, V. F. Razumov, H. Sitter, S. Bauer, and N. S. Sariciftci (2012) Indigo–a natural pigment for high performance ambipolar organic field effect transistors and circuits Adv. Mater. 24: 375–380.
Kong, K. W., H. E. Khoo, K. N. Prasad, A. Ismail, C. P. Tan, and N. F. Rajab (2010) Revealing the power of the natural red pigment lycopene Molecules. 15: 959–987.
Narsing Rao, M. P., M. Xiao, and W. J. Li (2017) Fungal and bacterial pigments: secondary metabolites with wide applications Front. Microbiol. 8: 1113.
Abed, R. M., S. Al Kharusi, A. Schramm, and M. D. Robinson (2010) Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman FEMS Microbiol. Ecol. 72: 418–428.
Venil, C. K., Z. A. Zakaria, and W. A. Ahmad (2013) Bacterial pigments and their applications Process Biochem. 48: 1065–1079.
Numan, M., S. Bashir, R. Mumtaz, S. Tayyab, N. U. Rehman, A. L. Khan, Z. K. Shinwari, and A. Al-Harrasi (2018) Therapeutic applications of bacterial pigments: a review of current status and future opportunities 3 Biotech. 8: 207.
Iwashina, T. (2015) Flavonoid properties in plant families synthesizing betalain pigments (review) Nat. Prod. Commun. 10: 1103–1114.
Chitra, G, D. S. Franklin, S. Sudarsan, M. Sakthivel, and S. Guhanathan (2017) Indole-3-acetic acid/diol based pH-sensitive biological macromolecule for antibacterial, antifungal and antioxidant applications Int. J. Biol. Macromol. 95: 363–375.
Shahid Ul, I. and B. S. Butola (2019) Effect of chitosan biological macromolecule on colorimetric analysis and radical scavenging activity of linen using pineapple peel extract biomolecules Int. J. Biol. Macromol. 124: 708–715.
Reichenbach, H., W. Kohl, A. Bottger-Vetter, and H. Achenbach (1980) Flexirubin-type pigments in Flavobacterium. Arch. Microbiol. 126: 291–293.
Achenbach, H., W. Kohl, W. Wachter, and H. Reichenbach (1978) Investigations of the pigments from Cytophaga johnsonae Cy jl. New flexirubin-type pigments Arch. Microbiol. 117: 253–257.
Kulandaisamy Venil, C., Z. A. Zakaria, R. Usha, and W. A. Ahma (2014) Isolation and characterization of flexirubin type pigment from Chryseobacterium sp. UTM-3T Biocatal. Agric. Biotechnol. 3: 103–107.
Venil, C. K., P. Sathishkumar, M. Malathi, R. Usha, R. Jayakumar, A. R. M. Yusoff, and W. A. Ahmad (2016) Synthesis of flexirubin-mediated silver nanoparticles using Chryseobacterium artocarpi CECT 8497 and investigation of its anticancer activity Mater. Sci. Eng C Mater. Biol. Appl. 59: 228–234.
Ji, H. F. (2010) Insight into the strong antioxidant activity of deinoxanthin, a unique carotenoid in Deinococcus radiodurans. Int. J. Mol. Sci. 11: 4506–4510.
Li, Y., H. Zhu, X. Lei, H. Zhang, C. Guan, Z. Chen, W. Zheng, H. Xu, Y. Tian, Z. Yu, and T. Zheng (2015) The first evidence of deinoxanthin from Deinococcus sp. Y35 with strong algicidal effect on the toxic dinoflagellate Alexandrium tamarense. J. Hazard. Mater. 290: 87–95.
Suresh, K., G. S. N. Reddy, S. Sengupta, and S. Shivaji (2004) Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India Int. J. Syst. Evol. Microbiol. 54: 457–461.
Lai, W. A., P. Kampfer, A. B. Aran, F. T. Shen, B. Huber, P. D. Rekha, and C. C. Young (2006) Deinococcus ficus sp. nov., isolated from the rhizosphere of Ficus religiosa L Int. J. Syst. Evol. Microbiol. 56: 787–791.
Wang, W., J. Mao, Z. Zhang, Q. Tang, Y. Xie, J. Zhu, L. Zhang, Z. Liu, Y. Shi, and M. Goodfellow (2010) Deinococcus wulumuqiensis sp. nov., and Deinococcus xibeiensis sp. nov., isolated from radiation-polluted soil Int. J. Syst. Evol. Microbiol. 60: 2006–2010.
Chaudhary, R., A. Gupta, S. Kota, and H. S. Misra (2019) N-terminal domain of DivIVA contributes to its dimerization and interaction with genome segregation proteins in a radioresistant bacterium Deinococcus radiodurans. Int. J. Biol. Macromol. 128: 12–21.
Seo, D. H., J. H. Jung, and C. S. Park (2019) Improved polymerization activity of Deinococcus geothermalis amylosucrase by semi-rational design: Effect of loop flexibility on the polymerization reaction Int. J. Biol. Macromol. 130: 177–185.
Lee, D., S. Cha, J. H. Jang, and T. Seo (2016) Deinococcus arenae sp. nov., a novel species isolated from sand in South Korea Antonie Van Leeuwenhoek. 109: 1055–1062.
Xu, X., L. Jiang, Z. Zhang, Y. Shi, and H. Huang (2013) Genome sequence of a gamma- and UV-ray-resistant strain, Deinococcus wulumuqiensis R12 Genome Announc. 1: e00206–13.
Hong, S., C. E. Farrance, A. Russell, and H. Yi (2015) Reclassification of Deinococcus xibeiensis Wang et al. 2010 as a heterotypic synonym of Deinococcus wulumuqiensis Wang etal. 2010 Int. J. Syst. Evol. Microbiol. 65: 1083–1085.
Bruch, E. M., A. de Groot, S. Un, and L. C. Tabares (2015) The effect of gamma-ray irradiation on the Mn(II) speciation in Deinococcus radiodurans and the potential role of Mn(II)-orthophosphates Metallomics. 7: 908–916.
Diaz, B. and D. Schulze-Makuch (2006) Microbial survival rates of Escherichia coli and Deinococcus radiodurans under low temperature, low pressure, and UV-Irradiation conditions, and their relevance to possible Martian life Astrobiology. 6: 332–347.
S. Namgung, H. A. Park, J. Kim, P. G. Lee, B. G. Kim, Y. H. Yang, and K. Y. Choi (2019) Ecofriendly one-pot biosynthesis of indigo derivative dyes using CYP102G4 and PrnA halogenase Dyes Pigm. 162: 80–88.
S. Y. Aim, M. Choi, D. Jeong, S. Park, H. Park, K. S. Jang, and K. Y. Choi (2019) Synthesis and chemical composition analysis of protocatechualdehyde-based novel melanin dye by 15T FT-ICR: High dyeing performance on soft contact lens Dyes Pigm. 160: 546–554.
Yabe, S., Y. Sakai, K. Abe, and A. Yokota (2017) Diversity of Ktedonobacteria with actinomycetes-like morphology in terrestrial environments Microbes Environ. 32: 61–70.
Tai, C. J., H. P. Kuo, F. L. Lee, H. K. Chen, A. Yokota, and C. C. Lo (2006) Chryseobacterium taiwanense sp. nov, isolated from soil in Taiwan Int. J. Syst. Evol. Microbiol. 56: 1771–1776.
Chaudhary, D. K. and J. Kim (2017) Chryseobacterium nepalense sp. nov., isolated from oil-contaminated soil Int. J. Syst. Evol. Microbiol. 67: 646–652.
Huq, M. A. (2018) Chryseobacterium chungangensis sp. nov., a bacterium isolated from soil of sweet gourd garden Arch. Microbiol. 200: 581–587.
Guo, W, J. Li, M. Shi, K. Yuan, N. Li, and G. Wang (2016) Chryseobacterium montanum sp. nov. isolated from mountain soil Int. J. Syst. Evol. Microbiol. 66: 4051–4056.
Xu, X., L. Tian, J. Xu, C. Xie, L. Jiang, and H. Huang (2018) Analysis and expression of the carotenoid biosynthesis genes from Deinococcus wulumuqiensis R12 in engineered Escherichia coli. AMB Express. 8: 94.
Chanudom, L. and J. Tangpong (2015) Anti-inflammation property of Syzygium cumini (L.) Skeels on indomethacin-induced acute gastric ulceration Gastroenterol Res. Pract. 2015: 343642.
Jimenez, M. E. P., C. M. B. Pinilla, E. Rodrigues, and A. Brandelli (2018) Extraction and partial characterisation of antioxidant pigment produced by Chryseobacterium sp. kr6 Nat. Prod. Res. 33: 1541–1549.
Ordenes-Aenishanslins, N., G. Anziani-Ostuni, M. Vargas-Reyes, J. Alarcon, A. Tello, and J. M. Perez-Donoso (2016) Pigments from UV-resistant Antarctic bacteria as photosensitizers in Dye Sensitized solar cells J. Photochem. Photobiol B. 162: 707–714.
Dzeha, T., C. Nyiro, D. Kardasopoulos, D. Mburu, J. Mwafaida, M. J. Hall, and J. G. Burgess (2018) UV resistance of bacteria from the Kenyan Marine cyanobacterium Moorea producens. Microbiologyopen. 8: e00697.
C. K. Venil, Z. A. Zakaria, R. Ush, and W. A. Ahmad (2014) Isolation and characterization of flexirubin type pigment from Chryseobacterium sp. UTM-3T Biocatal. Agric. Biotechnol. 3: 103–107.
Lysenko, V. S., V. A. Chistyakov, D. V. Zimakov, V. G. Soier, M. A. Sazykina, M. I. Sazykina, I. S. Sazykin, and V. P. Krasnov (2011) Separation and mass spectrometry identification of carotenoid complex from radioresistant bacteria Deinococcus radiodurans. J. Anal. Chem. 66: 1281–1284.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korea government (MEST) (2018R1D1A1B07046920).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations of Competing Interest The authors declare no conflict of interests.
Ethical Statement Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Park, S.A., Ahn, SY. & Choi, KY. Functional Microbial Pigments Isolated from Chryseobacterium and Deinococcus species for Bio-paint Application. Biotechnol Bioproc E 25, 394–402 (2020). https://doi.org/10.1007/s12257-019-0372-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0372-3