Abstract
A trinuclear copper(II) complex, namely [Cu3(L)2(CF3COO)2] (1) is afforded integrating a ‘compartmental’ Schiff base, H2L [where H2L = N,N′-bis(salicylidene)-1,3-propanediamine] and fully characterized. Complex 1 is implemented for electrocatalytic proton reduction to originate molecular hydrogen in a homogenous system. The electrochemical study involving CV (cyclic voltammetry), CPE (controlled potential electrolysis) and GC (gas chromatography) reveals that in homogeneous system, 1 is highly active towards proton reduction in presence of trifluoro acetic acid as a proton source. Following CPE at − 1.8 V vs Ag/AgCl, the headspace gas is sampled and gas chromatography is carried out to quantify the amount of hydrogen discharged. The ratio of theoretical H2 production from charge aggregation during electrolysis to the practical amount of H2 resolved on GC is yielded a Faradaic efficiency value of 87%. Moreover, an extended bulk electrolysis experiment carried out for 35 h illustrates the presence of a stable catalytic system in which 230 C of charge is accumulated. As a consequence, this efficient and stable H2 evolution obtained using a Cu(II) derivative (CuII is cheap and earth abundant) has shown that the synthesis of judiciously designed copper complex is a promising strategy towards potential catalysts from proton reduction.
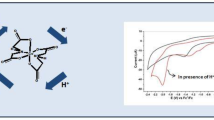
Graphic Abstract

Electrochemical and catalytic study of a trimetallic Cu(II) derivative in DMSO with the presence of trifluoroacetic acid as weak proton source shows the hydrogen evolution Faradaic efficiency as 87%. A continuous increment of the charge accumulation through time is observed, indicating the high stability of our catalyst under electrochemical H2 generation.







Similar content being viewed by others
References
Bigi JP, Hanna TE, Harman WH, Chang A, Chang CJ (2010) Chem Commun 46:958–960
Zhao F, Zhang J, Abe T, Wöhrle D, Kaneko M (1999) J Mol Cat A: Chem 145:245–256
Kellett RM, Spiro T (1985) Inorg Chem 24:2373–2377
Fisher BJ, Eisenberg R (1980) J Am Chem Soc 102:7361–7363
Beyene BB, Das K, Kerayu BA, Datta A, Hung CH (2019) Cat Commun 119:111–114
Beyene BB, Mane SB, Leonardus M, Hung CH (2017) ChemistrySelect 2:10565–10571
Beyene BB, Hung CH (2018) Electrocatalysis 9:689–696
DiRisio RJ, Armstrong JE, Frank MA, Lake WR, McNamara WR (2017) Dalton Trans 46:10418–10425
Beyene BB, Mane SB, Hung CH (2015) Chem Commun 51:15067–15070
Houlding V, Geiger T, Kolle U, Gratzel M (1982) J Chem Soc Chem Commun 12:681–683
Koelle U, Paul S (1986) Inorg Chem 25:2689–2694
Losse S, Vos JG, Rau S (2010) Coord Chem Rev 254:2492–2504
Artero V, Chavarot-Kerlidou M, Fontecave M (2011) Angew Chem Int Ed 50:7238–7266
Jacobsen GM, Yang JY, Twamley B, Wilson AD, Bullock RM, DuBois MR, DuBois DL (2008) Energy Environ Sci 1:167–174
Matsumura Y (2013) Int J Hydr Energy 38:13950–13960
Datta A, Das K, Beyene BB, Gajewska MJ, Garribba E, Hung CH (2017) Mol Cat 439:81–90
Felton GAN, Vannucci AK, Chen J, Lockett LT, Okumura N, Petro BJ, Zakai UI, Evans DH, Glass RS, Lichtenberger DL (2007) J Am Chem Soc 129:12521–12530
Darensbourg MY, Lyon EJ, Zhao X, Georgakaki IP (2003) Proc Nat Acad Sci 100:3683–3688
Tard C, Liu X, Ibrahim SK, Bruschi M, Gioia LD, Davies SC, Yang X, Wang LS, Sawers G, Pickett CJ (2005) Nature 433:610–613
Sun L, Åkermark B, Ott S (2005) Coord Chem Rev 249:1653–1663
Kaur-Ghumaan S, Schwartz L, Lomoth R, Stein M, Ott S (2010) Angew Chem Int Ed 122:8207–8211
Kaur-Ghumaan S, Schwartz L, Lomoth R, Stein M, Ott S (2010) Angew Chem Int Ed 49:8033–8036
Mejia-Rodriguez R, Chong D, Reibenspies JH, Soriaga MP, Darensbourg MY (2004) J Am Chem Soc 126:12004–12014
Canaguier S, Artero V, Fontecave M (2008) Dalton Trans 3:315–325
Dubois MR, Dubois DL (2009) Acc Chem Res 42:1974–1982
Dubois DL, Bullock RM (2011) Eur J Inorg Chem 2011:1017–1027
Collin JP, Jouaiti A, Sauvage JP (1988) Inorg Chem 27:1986–1990
Kilgore UJ, Roberts JAS, Pool DH, Appel AM, Stewart MP, Dubois MR, Dougherty WG, Kassel WS, Bullock RM, Dubois DL (2011) J Am Chem Soc 133:5861–5872
Helm ML, Stewart MP, Bullock RM, Dubois MR, Dubois DL (2011) Science 333:863–866
Natali M, Luisa A, Iengo E, Scandola F (2014) Chem Commun 50:1842–1844
Baffert C, Artero V, Fontecave M (2007) Inorg Chem 46:1817–1824
Sun Y, Bigi JP, Piro NA, Tang ML, Long JR, Chang CJ (2011) J Am Chem Soc 133:9212–9215
Jacques PA, Artero V, Pécaut J, Fontecave M (2009) Proc Natl Acad Sci 106:20627–20632
Dempsey JL, Brunschwig BS, Winkler JR, Gray HB (2009) Acc Chem Res 42:1995–2004
McNamara WR, Han Z, Alperin PJ, Brennessel WW, Holland PL, Eisenberg R (2011) J Am Chem Soc 133:15368–15371
Beyene BB, Mane SB, Hung CH (2018) J Electrochem Soc 165:H481–H487
Artero V, Chavarot-Kerlidou M, Fontecave M (2011) Angew Chem Int Ed 123:7376–7405
Das K, Beyene BB, Datta A, Garribba E, Hung CH (2018) Cat Lett 148:2703–2708
Beyene BB, Hung CH (2018) Sustain Energy Fuels 2:2036–2043
Zhang P, Wang M, Yang Y, Yao T, Sun L (2014) Angew Chem Int Ed 53:13803–13807
Zhao J, Tran PD, Chen Y, Loo JSC, Barber J, Xu ZJ (2015) ACS Cat 5:4115–4120
Sirbu D, Turta C, Gibson EA, Benniston AC (2015) Dalton Trans 44:14646–14655
Du J, Wang J, Ji L, Xu X, Chen Z (2016) ACS Appl Mat Int 8:30205–30211
Liu X, Cui S, Sun Z, Du P (2015) Chem Commun 51:12954–12957
Vaduva CC, Vaszilcsin N, Kellenberger A, Medeleanu M (2011) Int J Hydrogen Energy 36:6994–7001
Wang M, Chen L, Sun L (2012) Energy Env Science 5:6763–6778
Solis BH, Yu Y, Hammes-Schiffer S (2013) Inorg Chem 52:6994–6999
Marinescu SC, Winkler JR, Gray HB (2012) Proc Nat Acad Sci USA 109:15127–15131
Bullock RM, Appel AM, Helm ML (2014) Chem Commun 50:3125–3127
mL of a methanolic solution of Cu(CF3COO)2.4H2O (0.610 g, 2 mmol) were added to a a methanolic solution (10 mL) of [CuL] (1 mmol), under constant stirring. The resulting green solution was then kept boiling for 10 mins and then it was left undisturbed at room temperature. Dark-green square-shaped single crystals of 1 were generated after one week. These were separated by filtration and air-dried before X-ray diffraction analysis. Yield: 0.67 g. Anal. Calc. for C38H32N4O8F6Cu3: C, 46.66; H, 3.30; N, 5.73. Found: C, 46.89; H, 3.57; N, 5.59%.
Reglinski J, Morris S, Stevenson DE (2002) Polyhedron 21:2167–2174
Rahaman SH, Ghosh R, Lu TH, Ghosh BK (2005) Polyhedron 24:1525–1532
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds, theory and applications in inorganic chemistry, 5th edn. John Wiley and Sons Inc, New York
Crystal structure determination for 1: C38H32O8N4F6Cu3 (M = 977.29), monoclinic, space group P21/c, a = 9.553(1) Å, b = 10.105(1) Å, c = 19.128(2) Å, β = 94.607(2), V = 1840.5(3) Å3, Z = 2, Dc = 1.763 g cm-3, μ(MoKα) = 1.809 mm-1, F(000) = 986, 24398 reflections measured, of which 4364 were observed (I %3e 2σ(I)), R1 = 0.0515, wR2 = 0.1428, 266 parameters. CCDC-1493436.
Das K, Datta A, Massera C, Roma-Rodrigues C, Barroso M, Baptista PV, Fernandes AR (2019) J Coord Chem 72:920–940
Thakurta S, Chakraborty J, Rosair G, Tercero J, Fallah MSE, Garribba E, Mitra S (2008) Inorg Chem 47:6227–6235
Thakurta S, Rizzoli C, Butcher RJ, Gómez-García CJ, Garribba E, Mitra S (2010) Inorg Chim Acta 363:1395–1403
Saha S, Sasmal A, Choudhury CR, Gomez-Garcia CJ, Garribba E, Mitra S (2014) Polyhedron 69:262–269
Datta A, Das K, Mane SB, Mendiratta S, Fallah MSE, Garribba E, Bauzá A, Frontera A, Hung CH, Sinha C (2016) RSC Adv 6:54856–54865
Frisch MR, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, VZakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery, Jr. JA,, JPeralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox, Gaussian DJ, Inc., Wallingford CT (2016)
Becke AD (1993) J Chem Phys 98:5648–5652
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364–382
Barone V, In recent advances in density functional methods, Part I, Ed. D.P. Chong (World Scientific Publ. Co., Singapore, 1996).
Acknowledgements
KD expresses her appreciation to the SERB (Science and Engineering Research Board, India) Grant (PDF/2016/002832) for financial assistance. AD and CHH would like to express their appreciation to the Ministry of Science and Technology, Taiwan for financial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that thay have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Das, K., Beyene, B.B., Yibeltal, A.W. et al. A Trimetallic Cu(II) Derivative as an Efficient and Stable Electrocatalyst for Reduction of Proton to Molecular Hydrogen. Catal Lett 150, 2200–2207 (2020). https://doi.org/10.1007/s10562-020-03150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03150-x