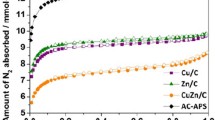
Abstract
Formic acid (FA) has attracted increasing interest in the utilization as a promising hydrogen carrier. Nitrogen-doped activated carbons were used as support to prepare well-dispersed Pd/C catalyst for hydrogen production from FA. The introduced N species adjusted the electronic properties of Pd and promoted dispersion of Pd, which were beneficial to improve the catalytic activity. Pd supported on the activated carbon aminated at 950 °C (the particle size of Pd is 2.8 ± 0.1 nm) showed the highest turnover frequency (TOF) of 1631 h−1 at 30 °C.
Graphic Abstract









Similar content being viewed by others
References
Eppinger J, Huang KW (2017) ACS Energy Lett 2:188–195
Grasemann M, Laurenczy G (2012) Energy Environ Sci 5:8171–8181
Singh AK (2016) Singh S Kumar A. Catal Sci Technol 6:12–40
Li Z, Xu Q (2017) Acc Chem Res 50:1449–1458
Zhou W, Li M, Ding OL, Chan SH (2014) Zhang L Xue Y. Int J Hydrog Energy 39:6433–6442
Zhang S, Jiang B (2017) Jiang K Cai WB. ACS Appl Mater Inter 9:24678–24687
Bandosz TJ (1999) Carbon 37:483–491
Deryło-Marczewska A, Goworek J (2004) Świątkowski A Buczek B. Carbon 42:301–306
Wang ZL, Yan JM, Wang HL (2013) Ping Y Jiang Q. J Mater Chem A 1:12721–12725
Sun J, Qiu H, Cao W, Fu H, Wan H (2018) Xu Z Zheng S. ACS Sustain Chem Eng 7:1963–1972
Mori K, Masuda S, Tanaka H, Yoshizawa K (2017) Che M Yamashita H. Chem Commun (Camb) 53:4677–4680
Zhu DJ, Wen YH, Xu Q (2017) Zhu QL Wu XT. Eur J Inorg Chem 2017:4808–4813
Chen H, Yang G, Feng Y, Shi C, Xu S (2012) Cao W Zhang X. Chem Eng J 198–199:45–51
Zhang W, Jiang X, Wang X, Kaneti YV, Chen Y, Liu J, Jiang JS (2017) Yamauchi Y Hu M. Angew Chem Int Ed Engl 56:8435–8440
Boudou JP, Chehimi M, Broniek E (2003) Siemieniewska T Bimer. J Carbon 41:1999–2007
Teschner D, Vass E, Havecker M, Zafeiratos S, Schnorch P, Sauer H, Knopgericke A, Schlogl R (2006) Chamam M Wootsch A. J Catal 242:26–37
Arrigo R, Schuster ME, Xie Z, Yi Y, Wowsnick G, Sun LL, Hermann KE, Friedrich M, Kast P, Hävecker M (2015) Knop-Gericke A Schlögl R. ACS Catal 5:2740–2753
Arrigo R, Schuster ME, Abate S, Wrabetz S, Amakawa K, Teschner D, Freni M, Centi G, Perathoner S (2014) Havecker M Schlogl R. Chemsuschem 7:179–194
Balmes O, Resta A, Wermeille D, Felici R, Messing ME, Deppert K, Liu Z, Grass ME, Bluhm H, van Rijn R, Frenken JW, Westerstrom R, Blomberg S, Gustafson J (2012) Andersen JN Lundgren E. Phys Chem Chem Phys 14:4796–4801
Wang F, Xu J, Shao X, Su X (2016) Huang Y Zhang T. Chemsuschem 9:246–251
Usachov D, Vilkov O, Gruneis A, Haberer D, Fedorov A, Adamchuk VK, Preobrajenski AB, Dudin P, Barinov A, Oehzelt M (2011) Laubschat C Vyalikh DV. Nano Lett 11:5401–5407
Zacharska M, Bulusheva LG, Lisitsyn AS, Beloshapkin S, Guo Y, Chuvilin AL, Shlyakhova EV, Podyacheva OY, Leahy JJ (2017) Okotrub AV Bulushev DA. Chemsuschem 10:720–730
Li J, Chen W, Zhao H, Zheng X, Wu L, Pan H, Zhu J (2017) Chen Y Lu. J J Catal 352:371–381
Bulushev DA, Zacharska M, Shlyakhova EV, Chuvilin AL, Guo Y, Beloshapkin S (2015) Okotrub AV Bulusheva LG. ACS Catal 6:681–691
Tedsree K, Li T, Jones S, Chan CW, Yu KM, Bagot PA, Marquis EA (2011) Smith GD Tsang SC. Nat Nanotechnol 6:302–307
Bulushev DA, Jia L (2012) Beloshapkin S Ross JR. Chem Commun 48:4184–4186
Jeon M, Han DJ, Lee KS, Choi SH, Han J, Nam SW, Jang SC, Park HS, Yoon CW (2016) Int J Hydrog Energy 41:15453–15461
Kim Y, Kim DH (2019) Appl Catal B-Environ 244:684–693
Hu C, Pulleri JK (2014) Ting SW Chan KY. Int J Hydrog Energy 39:381–390
Javaid R (2013) Kawasaki S i, Ookawara R, Sato K, Nishioka M, Suzuki A Suzuki TM. J Chem Eng Jpn 46:751–758
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant Number 2018YFB0604902). The funding source has no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, M., Liang, W., Chen, H. et al. Efficient Hydrogen Production from Formic Acid Using Nitrogen-Doped Activated Carbon Supported Pd. Catal Lett 150, 2377–2384 (2020). https://doi.org/10.1007/s10562-020-03141-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03141-y