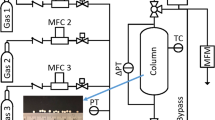
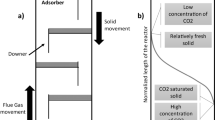
Abstract
The adsorption isotherms of water on Zeochem zeolite 13X were measured from 22 to 100 \(^{\circ }\text {C}\) and 0 to \(2.1\times 10^{-2}\) bar using volumetry and gravimetry. The equilibrium data was fit to a dual-site Langmuir isotherm. A series of single component H2O dynamic column breakthrough experiments were measured on zeolite 13X at \( 22\,^{\circ }\text {C}\) and 0.97 bar. These breakthrough experiments were modeled and simulated with our built in-house adsorption simulator. The simulator predicted composition and thermal breakthrough behavior well for all single component experiments. Competitive \(\text {CO}_{2}\)/\(\text {H}_{2}\text {O}\) breakthrough experiments were then performed at \( 22\,^{\circ }\text {C}\) and 0.99 bar. The collected equilibrium data showed up to a 98% loading reduction for \(\text {CO}_{2}\) (compared to the single component loading) for \(\approx \) 74.4% RH while \(\text {H}_{2}\text {O}\) showed no reduction compared to its single component loading. The binary equilibrium isotherms were described by an explicit water-loading adjusted dual-site Langmuir isotherm.







Similar content being viewed by others
Abbreviations
- b :
-
Adsorption equilibrium constant for site 1 (m\(^{3}\) mol\(^{-1}\))
- c :
-
Fluid phase concentration (mol m\(^{-3}\))
- \(C_\text {p}\) :
-
Heat capacity (J mol\(^{-1}\) K\(^{-1}\))
- d :
-
Adsorption equilibrium constant for site 2 (m\(^{3}\) mol\(^{-1}\))
- D :
-
Diffusivity (m\(^2\) s\(^{-1}\))
- h :
-
Heat transfer coefficient (W m\(^{-2}\) K\(^{-1}\))
- \(\Delta H\) :
-
Heat of adsorption (J mol\(^{-1}\))
- k :
-
Mass transfer coefficient (s\(^{-1}\))
- K :
-
Thermal conductivity (W m\(^{-1}\) K\(^{-1}\))
- L :
-
Length (m)
- m :
-
Adsorbent mass (kg)
- n :
-
Number of species (-)
- p :
-
Partial pressure (bar)
- P :
-
Total pressure (bar)
- q :
-
Solid phase loading (mol kg\(^{-1}\))
- \(q^*\) :
-
Equilibrium solid phase loading (mol kg\(^{-1}\))
- Q :
-
Outlet volumetric flow rate (m\(^3\) s\(^{-1}\))
- r :
-
Radius (m)
- R :
-
Universal gas constant (Pa m\(^{3}\) mol\(^{-1}\) K\(^{-1}\))
- t :
-
Time (s)
- \({\bar{t}}\) :
-
Dimensionless time (-)
- T :
-
Temperature (K)
- \(\Delta U\) :
-
Internal energy (J mol\(^{-1}\))
- v :
-
Interstitial velocity (m s\(^{-1}\))
- V :
-
Volume (m\(^{3}\))
- y :
-
Mole fraction (-)
- z :
-
Axial direction (m)
- \(\alpha\) :
-
Modified DSL saturation constant (kg mol\(^{-1}\))
- \(\beta\) :
-
Modified DSL nonlinearity constant (kg mol\(^{-1}\))
- \(\epsilon\) :
-
Bed voidage (-)
- \(\mu\) :
-
Viscosity (Pa s\(^{-1}\))
- \(\rho\) :
-
Adsorbent density (kg m\(^{-3}\))
- \(\tau\) :
-
Tortuosity (-)
- a:
-
Adsorbed phase
- acc:
-
Solid and fluid phase accumulation
- ads:
-
Adsorbent or adsorption
- amb:
-
Ambient
- ave:
-
Average
- b:
-
Bed or column
- comp:
-
Component
- d:
-
Extra-column
- des:
-
Desorption
- g:
-
Fluid phase
- i:
-
Index of species
- iso:
-
Isosteric
- in:
-
Inlet or internal
- j:
-
Index of species
- L:
-
Length or low
- m:
-
Molecular
- out:
-
Outlet or external
- p:
-
Particle
- s:
-
Solid phase
- sat:
-
Ultimate saturation
- tot:
-
Total
- w:
-
Wall
- z:
-
Axial direction
- 0:
-
Initial
- CCS:
-
Carbon capture and storage
- DCB:
-
Dynamic column breakthrough
- DSL:
-
Dual-site Langmuir isotherm
- DOE:
-
Department of energy
- MFC:
-
Mass flow controller
- MFM:
-
Mass flow meter
- MS:
-
Mass spectrometer
- PN:
-
Perfect negative pairing
- PP:
-
Perfect positive pairing
- PSA:
-
Pressure-swing adsorption
- PT:
-
Pressure transducer
- \(\Delta\)PT:
-
Differential pressure transducer
- RH:
-
Relative humidity
- RHM:
-
Relative humidity meter
- TGA:
-
Thermogravimetric analysis
- TC:
-
Thermocouple
- VEMC:
-
Virial excess mixing coefficient
- VSA:
-
Vacuum-swing adsorption
References
Ahn, H., Lee, C.: Adsorption dynamics of water in layered bed for air-drying TSA process. AIChE J. 49(6), 1601–1609 (2003)
Boot-Handford, M.E., Abanades, J.C., Anthony, E.J., Blunt, M.J., Brandani, S.: Carbon capture and storage update. Energy Environ. Sci. 7(1), 130–189 (2014)
Breck, D.W.: Zeolite Molecular Sieves, vol. 4. Wiley, New York (1974)
Bui, M., Adjiman, C.S., Bardow, A., Anthony, E.J., Boston, A., Brown, S., Fennell, P.S., Fuss, S., Galindo, A., Hackett, L.A., et al.: Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11(5), 1062–1176 (2018)
Farmahini, A.H., Krishnamurthy, S., Friedrich, D., Brandani, S., Sarkisov, L.: From crystal to adsorption column: challenges in multiscale computational screening of materials for adsorption separation processes. Ind. Eng. Chem. Res. 57, 15491–15511 (2018)
Ferreira, D., Magalhães, R., Taveira, P., Mendes, A.: Effective adsorption equilibrium isotherms and breakthroughs of water vapor and carbon dioxide on different adsorbents. Ind. Eng. Chem. Res. 50(17), 10201–10210 (2011)
Guntuka, S., Farooq, S., Rajendran, A.: A-and B-site substituted lanthanum cobaltite perovskite as high temperature oxygen sorbent. 2. column dynamics study. Ind. Eng. Chem. Res. 47(1), 163–170 (2008)
Haghpanah, R., Majumder, A., Nilam, R., Rajendran, A., Farooq, S., Karimi, I.A., Amanullah, M.: Multiobjective optimization of a four-step adsorption process for postcombustion CO\(_{2}\) capture via finite volume simulation. Ind. Eng. Chem. Res. 52(11), 4249–4265 (2013)
Hefti, M., Mazzotti, M.: Postcombustion CO\(_{2}\) capture from wet flue gas by temperature swing adsorption. Ind. Eng. Chem. Res. 57(45), 15542–15555 (2018)
Hefti, M., Marx, D., Joss, L., Mazzotti, M.: Adsorption equilibrium of binary mixtures of carbon dioxide and nitrogen on zeolites ZSM-5 and 13X. Microporous Mesoporous Mater. 215, 215–228 (2015)
IPCC: Special report on carbon capture and storage. Technical report, Intergovernmental Panel on Climate Change (IPCC) (2005)
Joos, L., Swisher, J.A., Smit, B.: Molecular simulation study of the competitive adsorption of H\(_{2}\)O and CO\(_{2}\) in zeolite 13X. Langmuir 29(51), 15936–15942 (2013)
Kim, J., Lee, C., Kim, W., Lee, J., Kim, J., Suh, J., Lee, J.: Adsorption equilibria of water vapor on alumina, zeolite 13X, and a zeolite X/activated carbon composite. J. Chem. Eng. Data 48(1), 137–141 (2003)
Kim, H., Cho, H.J., Narayanan, S., Yang, S., Furukawa, H., Schiffres, S., Li, X., Zhang, Y., Jiang, J., Yaghi, O.M.: Characterization of adsorption enthalpy of novel water-stable zeolites and metal-organic frameworks. Sci. Rep. 6, 19097 (2016)
Kim, K., Oh, H., Lim, S., Ho, K., Park, Y., Lee, C.: Adsorption equilibria of water vapor on zeolite 3A, zeolite 13X, and dealuminated Y zeolite. J. Chem. Eng. Data 61(4), 1547–1554 (2016)
Krishnamurthy, S., Haghpanah, R., Rajendran, A., Farooq, S.: Simulation and optimization of a dual-adsorbent, two-bed vacuum swing adsorption process for CO\(_{2}\) capture from wet flue gas. Ind. Eng. Chem. Res. 53(37), 14462–14473 (2014)
Lemmon, E. W., Huber, M. L., McLinden, M. O.: NIST standard reference database 23: Reference fluid thermodynamic and transport properties-REFPROP, version 9.1. NIST Pubs, (2013)
Li, G., Xiao, P., Webley, P., Zhang, J., Singh, R., Marshall, M.: Capture of CO\(_{2}\) from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption 14(2–3), 415–422 (2008)
Lorek, A., Majewski, J.: Humidity measurement in carbon dioxide with capacitive humidity sensors at low temperature and pressure. Sensors 18(8), 2615 (2018)
Purdue, M.J.: Explicit flue gas adsorption isotherm model for zeolite 13X incorporating enhancement of nitrogen loading by adsorbed carbon dioxide and multi-site affinity shielding of co-adsorbate dependent upon water vapor content. J. Phys. Chem. C 122, 11832–11847 (2018)
Purdue, M.J., Qiao, Z.: Molecular simulation study of wet flue gas adsorption on zeolite 13X. Microporous Mesoporous Mater. 261, 181–197 (2018)
Rajagopalan, A.K., Avila, A.M., Rajendran, A.: Do adsorbent screening metrics predict process performance? A process optimisation based study for post-combustion capture of CO\(_{2}\). Int. J. Greenh. Gas Control 46, 76–85 (2016)
Rezaei, F., Rownaghi, A.A., Monjezi, S., Lively, R.P., Jones, C.W.: SO\(_{{\rm x}}\)/NO\(_{{\rm x}}\) removal from flue gas streams by solid adsorbents: a review of current challenges and future directions. Energy Fuels 29(9), 5467–5486 (2015)
Ribeiro, A.M., Sauer, T.P., Grande, C.A., Moreira, R.F.P.M., Loureiro, J.M., Rodrigues, A.E.: Adsorption equilibrium and kinetics of water vapor on different adsorbents. Ind. Eng. Chem. Res. 47(18), 7019–7026 (2008)
Ritter, J.A., Bhadra, S.J., Ebner, A.D.: On the use of the dual-process Langmuir model for correlating unary equilibria and predicting mixed-gas adsorption equilibria. Langmuir 27(8), 4700–4712 (2011)
Ritter, J.A., Bumiller, K.C., Tynan, K.J., Ebner, A.D.: On the use of the dual process Langmuir model for binary gas mixture components that exhibit single process or linear isotherms. Adsorption 25, 1511–1523 (2019)
Ryu, Y.K., Lee, S.J., Kim, J.W., Leef, C.: Adsorption equilibrium and kinetics of H\(_{2}\)O on zeolite 13X. Korean J. Chem. Eng. 18(4), 525–530 (2001)
Sircar, S., Cao, D.V.: Heat of adsorption. Chem. Eng. Technol. 25(10), 945–948 (2002)
Stern, N.: The economics of climate change. Am. Econ. Rev. 98(2), 1–37 (2008)
Wang, Y., LeVan, M.D.: Adsorption equilibrium of carbon dioxide and water vapor on zeolites 5A and 13X and silica gel: pure components. J. Chem. Eng. Data 54(10), 2839–2844 (2009)
Wang, Y., LeVan, M.D.: Adsorption equilibrium of binary mixtures of carbon dioxide and water vapor on zeolites 5A and 13X. J. Chem. Eng. Data 55(9), 3189–3195 (2010)
Wilkins, N.S., Rajendran, A.: Measurement of competitive \(\text{CO}_{2}\) and \(\text{ N}_{2}\) adsorption on zeolite 13X for post-combustion \(\text{ CO}_{2}\) capture. Adsorption 25(2), 115–133 (2019)
Xiao, P., Zhang, J., Webley, P., Li, G., Singh, R., Todd, R.: Capture of CO\(_{2}\) from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption 14(4–5), 575–582 (2008)
Acknowledgements
Funding support from the Canada Foundation for Innovation John R. Evans Leaders Fund Project Number 33801 and Canada First Excellence Fund through University of Alberta Future Energy Systems are acknowledged. We thank Zeochem for providing samples of the zeolite 13X used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilkins, N.S., Sawada, J.A. & Rajendran, A. Measurement of competitive \(\text {CO}_{2}\) and \(\text {H}_{2}\text {O}\) adsorption on zeolite 13X for post-combustion \(\text {CO}_{2}\) capture. Adsorption 26, 765–779 (2020). https://doi.org/10.1007/s10450-020-00199-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-020-00199-3