Abstract
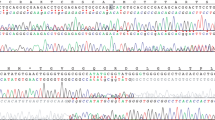
Gorham–Stout disease (GSD), a rare disorder of unknown etiology, is characterized by massive osteolysis that is associated with proliferation and dilation of lymphatic vessels. Variants in cancer-associated genes have been described in complex lymphatic anomalies. To explore the pathogenesis of GSD, we performed the amplicon-based deep sequencing on 50 cancer-related genes to assay affected tissues from the six patients with GSD. In one patient, a somatic activating KRAS c.182A > G variant (p.Q61R) was detected in 1% of the tissue sample. Conversely, the mutant allele was not detected in uninvolved normal skin and blood samples. Histopathology of the patient’s tissue sample showed proliferation of abnormal lymphatic and blood vascular endothelial cells, osteoclasts, and activated macrophages. The activating KRAS variant is a known ‘hotspot’ variant, frequently identified in several types of human cancer. This is the first report of identifying a pathogenic variant in a patient with GSD. This finding may set the stage for elucidation of pathophysiology and the development of novel therapies for GSD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Trenor CC 3rd, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23:186–90.
Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham–Stout disease. Bone. 2014;63:47–52.
Manevitz-Mendelson E, Leichner GS, Barel O, Davidi-Avrahami I, Ziv-Strasser L, Eyal E, et al. Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis. 2018;21:287–98.
Barclay SF, Inman KW, Luks VL, McIntyre JB, Al-Ibraheemi A, Church AJ, et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet Med. 2019;21:1517–24.
Ozeki M, Aoki Y, Nozawa A, Yasue S, Endo S, Hori Y, et al. Detection of NRAS mutation in cell-free DNA biological fluids from patients with kaposiform lymphangiomatosis. Orphanet J Rare Dis. 2019;14:215.
Rodriguez-Laguna L, Agra N, Ibañez K, Oliva-Molina G, Gordo G, Khurana N, et al. Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly. J Exp Med. 2019;216:407–18.
Muñoz-Maldonado C, Zimmer Y, Medová M. A comparative analysis of individual RAS mutations in cancer biology. Front Oncol. 2019;9:1088.
Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82.
Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55:331–43.
Nozawa A, Ozeki M, Hori T, Kato H, Ohe N, Fukao T. Fatal progression of Gorham–Stout Disease with skull base osteomyelitis and lateral medullary syndrome. Intern Med. 2019;58:1929–33.
Aoki Y, Niihori T, Inoue S, Matsubara Y. Recent advances in RASopathies. J Hum Genet. 2016;61:33–39.
Nguyen HL, Boon LM, Vikkula M. Vascular anomalies caused by abnormal signaling within endothelial cells: targets for novel therapies. Semin Intervent Radio. 2017;34:233–8.
Li QF, Decker-Rockefeller B, Bajaj A, Pumiglia K. Activation of Ras in the vascular endothelium induces brain vascular malformations and hemorrhagic stroke. Cell Rep. 2018;24:2869–82.
Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med. 2018;378:250–61.
Priemer DS, Vortmeyer AO, Zhang S, Chang HY, Curless KL, Cheng L. Activating KRAS mutations in arteriovenous malformations of the brain: frequency and clinicopathologic correlation. Hum Pathol. 2019;89:33–39.
Siegel DH, Cottrell CE, Streicher JL, Schilter KF, Basel DG, Baselga E, et al. Analyzing the genetic spectrum of vascular anomalies with overgrowth via cancer genomics. J Investig Dermatol. 2018;138:957–67.
Ozeki M, Fukao T. Generalized lymphatic anomaly and Gorham–Stout disease: overview and recent insights. Adv Wound Care. 2019;8:230–45.
Colucci S, Taraboletti G, Primo L, Viale A, Roca C, Valdembri D, et al. Gorham–Stout syndrome: a monocyte-mediated cytokine propelled disease. J Bone Min Res. 2006;21:207–18.
Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–80.
Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4.
Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–9.
Dias Carvalho P, Guimarães CF, Cardoso AP, Mendonça S, Costa ÂM, Oliveira MJ, et al. KRAS oncogenic signaling extends beyond cancer cells to orchestrate the microenvironment. Cancer Res. 2018;78:7–14.
Franco-Barrera MJ, Zavala-Cerna MG, Aguilar-Portillo G, Sánchez-Gomez DB, Torres-Bugarin O, Franco-Barrera MA, et al. Gorham–Stout disease: a clinical case report and immunological mechanisms in bone erosion. Clin Rev Allergy Immunol. 2017;52:125–32.
Illeez OG, Ozkan K, Ozkan FU, Bostan AB, Akpinar F, Bilgic B, et al. Zoledronic acid: treatment option for Gorham–Stout disease. Orthopade. 2018;47:1032–5.
Adams DM, Trenor CC 3rd, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137:e20153257.
Acknowledgements
We thank Dr. Saori Endo, Dr. Shiho Yasue, Dr. Akihito Nagano, and Dr. Toshiyuki Fukao of Gifu University, Dr. Yasuyuki Shibuya of Nagoya City University Hospital, Dr. Koji Yokoyama of Japanese Red Cross Wakayama Medical Center, Dr. Masataka Takahashi of the University of Tokyo Hospital, and Dr. Mitsuharu Fukazawa of Tagawa Municipal Hospital for their cooperation in the data analyses. We thank Dr. Koki Nagai, Dr. Taiki Abe, Ms. Yoko Tateda in Tohoku University for providing technical assistance of NGS analysis. We also acknowledge the technical support of the Biomedical Research Core of Tohoku University Graduate School of Medicine. We would like to thank Editage (www.editage.com) for English language editing.
Funding
The present study was supported partly by the Clinical Research-Clinical Trial Promotion Research Project (18lk0201055h0003), Practical Research Project for Rare/Intractable Diseases (18ek0109277h0002 and 18ek0109278h0002) from Japan’s Agency for Medical Research and Development (AMED), and a research grant from the Naito Foundation.
Author information
Authors and Affiliations
Contributions
AN, MO, and YA conceived the study and participated in its design. AN, TN, and YA performed the NGS and genetic analysis of the patients. NS and TM performed the pathological analysis. AN, MO, and YA wrote the paper. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committees of the Gifu University School of Medicine and Tohoku University School of Medicine.
Informed consent
Informed consent was obtained from the patients or their legal guardians for their inclusion in the study and subsequent publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nozawa, A., Ozeki, M., Niihori, T. et al. A somatic activating KRAS variant identified in an affected lesion of a patient with Gorham–Stout disease. J Hum Genet 65, 995–1001 (2020). https://doi.org/10.1038/s10038-020-0794-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-020-0794-y
This article is cited by
-
Analysis of circulating osteoclast and osteogenic precursors in patients with Gorham-Stout disease
Journal of Endocrinological Investigation (2024)
-
Genomic profiling informs diagnoses and treatment in vascular anomalies
Nature Medicine (2023)
-
Gorham-Stout case report: a multi-omic analysis reveals recurrent fusions as new potential drivers of the disease
BMC Medical Genomics (2022)
-
Primary lymphoedema
Nature Reviews Disease Primers (2021)