Abstract
Loss of drilling fluid commonly known as mud loss is considered as one of the critical issues during the drilling operation as it can cause severe formation damage. To minimize fluid loss, researchers introduced numerous additives but did not get the expected result. Recently, the use of nanoparticles (NPs) in drilling fluid gives a new hope to control the fluid loss. A basic KCl–Glycol–PHPA polymer-based mud is made, and six different concentrations of 0.1, 0.5, 1.0, 1.5, 2.0, 3.0 wt% iron (III) oxide or Hematite (Fe2O3) NPs are mixed with the basic mud. The experimental observations reveal that fluid loss of basic mud is 5.9 ml after 30 min and prepared nano-based drilling mud results in a less fluid loss at all concentrations. Nanoparticles with a concentration of 0.5 wt% result in a 5.1 ml fluid loss at the API LTLP filter press test. On the other hand, nanoparticles with a concentration of 3.0 wt% enhance the plastic viscosity, yield point, and 10 s gel strength by 15.0, 3.0, and 12.5%, respectively. The optimum concentration of hematite NPs is found to be 0.5 wt% which reduces the API LPLT filtrate volume and filter cake thickness by 13.6 and 40%, respectively, as well as an improvement of plastic viscosity by 10%.
Similar content being viewed by others
Introduction
Petroleum industry drives modern civilization by providing oil and gas like blood in the human body. The growth of human civilization and the necessities of livelihood with time forced the petroleum engineers to explore and produce a large amount of oil and gas. But at the same time, limited conventional oil and gas reserves are declining, which promotes the engineers to explore oil and gas from geologically complex formations. Geologically complex formation is associated with high temperature and high pressure. In these formations, drilling fluid plays crucial functions in cooling and lubricating drill bit, transporting and removing cutting efficiently, maintaining wellbore strength and making mud cake (Caenn and Chillingar 1996; Darley and Gray 1988). Approximately 60% of the well-drilling program is conducted using water-based mud (WBM), although WBM deteriorates at elevated temperature and elevated pressure conditions. On the other hand, oil-based muds (OBMs) show superior functionality compared to WBMs in terms of lubricity, corrosiveness, shale swelling and stability at lower and higher temperatures reservoir conditions (Growcock and Patel 2011). However, the restriction imposed in the application of OBMs to some areas, such as arctic and offshore drilling (Neff 2010) due to their environmental complication, high costs and safety issues (Patel et al. 2007). Thus, the cost of drilling mud which is approximately 5–20% of the total drilling cost (Lake 2007) may increase abruptly if drilling fluid and its properties are not maintained properly.
In drilling operation, majority of the problems encountered are directly or indirectly associated with the drilling fluid properties. (Hossain and Al-Majed 2015; M. E. Hossain and Islam 2018). Drilling fluid-related obstacles such as drilling rate, borehole instability, formation damage, shale swelling, stuck pipe, loss circulation, and fluid loss, etc. are common in the drilling operation (Beg et al. 2019; Elmousalami and Elaskary 2020; Kang et al. 2012). Drilling fluid invasion into the hydrocarbon-bearing zones can alter the permeability, wettability and capillary pressure which is essential to achieving desired productivity rates (Dandekar 2013). For this reason, drilling fluid systems are designed to preserve the virginity of the pay zones. Typical formation pore throat size lies in micro-scale and conventionally used loss circulating materials (LCM) are also in micro-scale which could not restrict the fluid invasion properly (Nelson 2009).
Recently, nanoparticles showed a significant impact on almost every engineering aspect because of its superior physical and chemical properties. To drill a more challenging well, the use of nanoparticles in drilling fluids may overcome the problems associated with the current drilling fluid systems (Al-Yasiri and Al-Sallami 2015). NPs with micro-sized conventional LCM can make bridges resulting a reduced void of inter-particle space in mud cake which prevent fluid flow from borehole to formation. Researchers introduced several nanoparticles in drilling fluid including ZnO, Montmorillonite, Carbon nanotubes, SiO2, Nanoclay, Nanographite, TiO2, Fe2O3, Graphene, Calcium oxide, CuO, Al2O3, etc. to enhance the fluid loss control and rheology (Al-saba et al. 2018; Aramendiz et al. 2019; Ismail et al. 2016; Jung et al. 2011; Mahmoud et al. 2018; Medhi et al. 2019; Mohammed 2017; Perween et al. 2018; Zakaria et al. 2012; Zamani et al. 2018). Previously, researchers tested Iron-based nanoparticles either alone or with other nanoparticles for the justification of filtration loss control. Jung et al. (2011) compared the results obtained using two different sized (3 and 30 nm) iron oxide (Fe2O3) nanoparticles at different concentrations (0.5 and 5 wt%). They found that for NPs of 3 nm size resulted 2.3 and 20% increment in API LTLP fluid loss at concentrations of 0.5 and 5 wt%, respectively. While for 30 nm, fluid loss reduction was 6.14% and increment was 14.5% at concentrations of 0.5 and 5 wt%, respectively. In terms of viscosity and yield stress, they found that NPs concentration and size has positive impact. At 5 wt% of NPs concentration, viscosity and yield stress increased compared to control fluid. Moreover, at 5 wt%, concentration of 3 nm size particles has superior impact than 30 nm size particles. While Barry et al. (2015) recorded 11.5 and 2.1% increment in fluid loss at concentrations of 0.5% for 3 and 30 nm size NPs, respectively. Vryzas et al. (2015) enhanced the filtration properties of bentonite-WBM by dosing iron oxide nanoparticles. Their investigation revealed that the enhancement in the filtration properties is optimum if the concentration of iron oxide nanoparticles is kept at 0.5 wt%. They presented that for NPs size < 50 nm LTLP fluid loss reduced 11.9, 17.4 and 19.3% at concentrations of 0.5, 1.5 and 2.5 wt%, respectively. Mahmoud et al. (2016) tested 0.3, 0.5, 1.5 and 2.5 wt% Fe2O3 NPs in WBM. They concluded that 0.5 wt% of NPs concentration resulted a considerable improvement in yield point while small increment in plastic viscosity compared to base fluid. They found that increasing NPs concentrations resulted in a more stable apparent viscosity. Mahmoud et al. (2018) found a 3.9% reduction in filtrate volume and 21.9% reduction in cake thickness at NPs concentration of 0.5 wt% under HTHP conditions. The investigations regarding the impact of ferric oxide NPs on WBM are limited. Moreover, most of the researchers investigated the impact of NPs on fluid loss control by mixing it with simple bentonite-WBM. But in a real situation, bentonite-WBM hardly used in reservoir zone. In this research, NPs are used in KCl–Glycol–PHPA polymer-based mud, because this mud system can efficiently control the wellbore shales swelling and encapsulate the drilling cuttings with a film that retards the dispersion and disintegration of solids (Clark et al. 1976). This paper presents the experimental analysis on the implementation of Hematite (Fe2O3) NPs in KCl–Glycol–PHPA mud system to enhance the fluid loss control and rheology. To the best of our knowledge, this is the first approach to dose hematite (Fe2O3) NPs in KCl–Glycol–PHPA WBM system. However, comparisons of iron oxide nanoparticles dosed in bentonite-WBM (previous) and KCl–Glycol–PHPA WBM (current study) are presented in Table 1.
Experimental section
All the experiments for this article were conducted in the laboratory. American Petroleum Institute (API) recommended practice API RP 13B-1 for testing water-based drilling fluid was followed to perform all drilling fluid test. Figure 1 illustrates the workflow of experiment considered in this study.
Materials selection
Ferric chloride hexahydrate (97% extra pure), and ammonia solution (25%) were purchased. Deionized water was prepared using a laboratory water distillation apparatus. All the drilling fluid additives were supplied from Bangladesh Petroleum Exploration and Production Company Ltd. (BAPEX).
Synthesis of iron (III) oxide nanoparticles
In this study, chemical precipitation technique was adopted to synthesize the iron (III) oxide (Fe2O3) nanoparticles. To synthesize nanoparticle, 5.41 g of ferric chloride hexahydrate (FeCl3.6H2O) was dissolved in 100 ml of deionized water to prepare a 0.2 M precursor and stirred 30 min at 80 °C with a magnetic stirrer. Then, 3 M of NH4OH was added to solution dropwise while stirring vigorously at ambient conditions. After 2.5–3.0 h of continuous stirring, the obtain product was allowed to precipitate completely. The resulting precipitate was subsequently filtered and washed with deionized water and ethanol to remove the excess base. Then it was dried in a hot air oven at 80 °C for 1.5 h. The product was then chopped into pieces which were further calcined in a muffle furnace at 400 °C for 4 h. Finally, a reddish-brown powder (Hematite) product was obtained which was characterized using X-ray powder diffraction (XRD).
Preparation of drilling fluid
Table 2 represents the list of all additives with the amount that were used to prepare base KCl–Glycol–PHPA polymer mud. For proper mixing of drilling fluid additives, it is necessary to ensure adding the additive sequentially presented in Table 2. A Hamilton Beach mixer with 36,000 rpm was used to mix all drilling fluid additives. After subsequently dosing all the additives, mixing continued for 20 min. Figure 2 shows the picture of Hamilton beach mixture during the preparation of mud. After preparing base mud, hematite NP-based KCl–Glycol–PHPA muds were prepared in varying concentrations of 0.1, 0.5, 1, 1.5, 2, and 3% by weight/volume. To prepare NP-based mud, the additives were added in the following sequences:
Water → Hematite nanoparticles → Soda ash → KCl → PAC-LV → PAC-RV → POLY-SAL → Xanthan gum → Barite →Sulphonated asphalt →Caustic potash → Biocide → Glydril MC → PHPA.
Measurement of rheology
Rheological investigation of each sample mud was performed at standard test temperature of 120 °F by using a Fann viscometer (Model 35SA). Equation 1 was adopted for the calculation of plastic viscosity (PV) of each sample mud. Yield points (YP) were computed using Eq. 2. Initial gel strength (GS) or 10 s Gel and final gel strength (GS) or 10 min Gel were obtained from viscometer dial reading in lbs/100ft2.
where
PV = Plastic viscosity (centipoise)
YP = Yield points (lb/100ft2)
Measurement of fluid loss
Filtration properties were investigated using a Fann API LTLP filter test equipment. Metal cylinder of the equipment was properly filled with drilling fluid and 100 psig pressure was applied from the top using CO2 charge. A graduated cylinder was kept below the metal cylinder. The volume of fluid collected in the graduated cylinder was considered as the filtrate or fluid loss. Fluid loss was measured for 30 min at ambient temperature. After performing 30 min filter test, the thickness of filter cake formed over filter paper was measured.
Results and discussion
Characterization of hematite nanoparticles
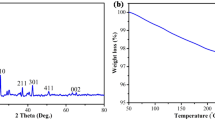
The X-ray powder diffraction pattern of the synthesized hematite nanoparticles is shown in Fig. 3. The XRD analysis of synthesized NPs demonstrates the characteristics pattern of α-Fe2O3 (JCPDS No. 33–0664). The characteristic peaks appear at following 2θ ranges ≈ 24.12°, 33.14°, 35.63°, 40.87°, 44.59°, 49.47°, 54.08°, 57.58°, 62.48°, and 64.05° which corresponds to 012, 104, 110, 113, 202, 024, 116, 018, 214, and 300 planes. From Debye–Scherrer formula, the crystal size of synthesized hematite nanoparticle was computed to be about 40 nm. From XRD analysis the crystallinity of synthesized NPS was found 76.46%.
Effect of hematite nanoparticles on rheology
The measurement of drilling fluid rheology demonstrates shear thinning behavior that means increasing shear rate causes a decrement in viscosity, presented in Fig. 4. At low shear rate, increasing NPs concentration shows higher viscosity compared to basic mud while at high shear rate, nano-based mud and basic mud show similar viscous behavior. It reveals a significant property of drilling fluid as high viscosities are essential during static conditions (tripping time) and low viscosities during dynamic conditions (drilling operation) to remove cuttings from the bottom hole (Chenevert and Sharma 2014; Fraser et al. 2003). The rheological flow behavior of NP-based mud and without NPs follow non-Newtonian fluid pattern. Figure 5 shows that shear stress versus shear rate diagram is best fitted for Bingham plastic model. Increasing NPs concentrations, the shear stress increases for the same shear rate. Plastic viscosity (PV) is defined as the flow resistance of fluid. Mechanical friction between solids, the liquids and the deformation of liquid due to shear stress, within the mud system are responsible for the plastic viscosity (Perween et al. 2018). For efficient drilling operation, this important mud property must be maintained within the desired ranges. Figure 6 shows that plastic viscosity initially does not show any change at low NPs concentration, but at higher concentration plastic viscosity enhances. Particularly NPs with concentration of 0.5 wt% gives 10% enhancement in plastic viscosity compare to base mud. Though 3 wt% NPs provides 15% enhancement in plastic viscosity. NPs increase the plastic viscosity because it contributes to the total dispersed solid in mud system.
Proper yield point (YP) is essential for drilling fluid cutting transport capacity. YP determines the drilling fluid capability to remove the drilling cuttings from beneath the drill bit to surface through annulus (Ismail et al. 2016). Normally, a high YP is expected to perform better cutting transport throughout the circulation system. From Fig. 7, it is found that YP increases with higher dosages of NPs. An increase of 3% in YP is observed when 3 wt% of NPs are used in base mud. Increased NPs concentration demonstrated improved yield point which will provide better cutting removal from borehole to surface. While only a 6.25% reduction in YP occurs at 0.5 wt% of NPs concentration although the resulted YP value is in satisfactory limit. This can be explained by the positive surface charge of iron oxide nanoparticles in aqueous state (Barry et al. 2015). At concentration of 0.5 wt%, the number of iron oxide particles are saturated enough that can exhibit large repulsive forces among particles resulting less coagulation and flocculation thereby lowering the yield point. While at higher concentrations of NPs, the reduction of inter-particle space is evident which limits the repulsion ability and promotes the flocculation producing a high YP (McElfresh et al. 2012).
Gel strength of mud reveals its cutting and weighting material holding capacity during static intervals. In other words, gel strength and yield stress are naturally the same except that gel strength is measured in static condition whereas yield stress is measured in a dynamic mode. Figure 8 represents the effect of NPs concentrations on 10 s and 10 min gel strength of nano-based drilling fluid. From figure, it reveals that at an optimum concentration of 0.5 wt% of NPs, 10 s gel strength is increased by 12.5% compared to base mud while in case of 10 min gel strength it remains approximately unchanged. For NPs, concentration of 1–2 wt% 10 min gel is decreased compared to base mud.
Effect of hematite nanoparticles on API fluid Loss
The impact of hematite NP concentrations on fluid loss is shown in Figs. 9 and 10. For all NP concentrations, API fluid loss reduces compared to base mud which represents considerable capability of NPs to control the fluid loss. For 30 min API LTLP filter test, in case of base mud, 5.9 ml of filtrate is obtained. On the other hand, NP concentration of 0.5 wt% produces 5.1 ml of filtrate which represents 13.6% enhancement in fluid loss control compared to base mud. From experimental observation the cumulative fluid loss is found minimum (5.1 ml) at NPs concentration of 0.5 wt%. While at higher NP concentrations, the cumulative fluid losses are found slightly higher (5.2–5.3 ml) which confirms the best performance of hematite nanoparticle at an optimum concentration of 0.5 wt%. This value is much closer to the value reported by the previous experimental work (Vryzas et al. 2015). It can be concluded that at a concentration of 0.5 wt%, the quantity of NPs is sufficient to block the pore space of filter paper. While at higher concentrations, the NP volume compared to LCM increases and affects to build proper bridge resulting slightly increased in filtrate volume.
Effect of hematite nanoparticles on mud cake thickness
The mud cake thickness for basic mud is measured 2/32 inch. While NP-based muds develop lower thickness at all concentrations compare to basic mud. Figure 11a, b represents the resulted mud cake thickness and the photos of mud cake obtained for different NPs concentrations under static condition, respectively. A 40% reduction in mud cake thickness is found for both 0.1 and 0.5 wt% NPs concentrations compared to base mud (2/32 inch). Thin mud cake is desired for drilling operation, because it could solve the differential stacking problem. Moreover, thin mud cake causes less fluid invasion into the formation which reflects less formation damage (Dehghani et al. 2019). Experimental investigation reveals that the optimum concentration of 0.5 wt% NPs results lowest filtrate volume 5.1 ml (explained in previous section) as well as thinnest mud cake (1.2/32 inch). This indicates that hematite NPs with a concentration of 0.5 wt% in KCl–Glycol–PHPA mud system would be used to reduce the formation damage during drilling.
Conclusions
-
a)
At low shear rate, nano-based mud demonstrates higher viscosities compared to base mud.
-
b)
API fluid filter test shows that the optimum concentration of Hematite is 0.5 wt% for the fluid loss control, which reduces 13.6% in filtrate volume compared to base mud.
-
c)
At NPs concentration of 0.5 wt%, the plastic viscosity and 10 s gel strength of mud are enhanced by 10 and 12.5%, respectively.
-
d)
At the optimum concentration of 0.5 wt%, the mud cake thickness is reduced by 40% compared to base mud.
-
e)
From observation, it can be concluded that hematite NPs with a concentration of 0.5 wt% in KCl–Glycol–PHPA mud system would be used in drilling system to reduce the formation damage.
-
f)
The experimental results confirmed that the iron oxide nanoparticles can also be used as a potential additive in KCl–Glycol–PHPA WBM system to reduce the fluid loss and mud cake thickness at all NP concentrations without any detrimental change of rheological properties.
References
Al-saba MT, Al Fadhli A, Marafi A, Hussain A, Bander F, Al Dushaishi MF (2018) Application of nanoparticles in improving rheological properties of water based drilling fluids. In: SPE Kingdom of Saudi Arabia snnual technical symposium and exhibition, Dammam. https://doi.org/10.2118/192239-MS
Al-Yasiri MS, Al-Sallami WT (2015) How the drilling fluids can be made more efficient by using nanomaterials. Am J Nano Res Appl 3:41–45. https://doi.org/10.11648/j.nano.20150303.12
Aramendiz J, Imqam AH, Fakher SM (2019) Design and evaluation of a water-based drilling fluid formulation using SiO and Graphene Oxide nanoparticles for unconventional shales. In: International petroleum technology conference, Beijing. https://doi.org/10.2523/IPTC-19342-MS
Barry MM, Jung Y, Lee J-K, Phuoc TX, Chyu MK (2015) Fluid filtration and rheological properties of nanoparticle additive and intercalated clay hybrid bentonite drilling fluids. J Petrol Sci Eng 127:338–346. https://doi.org/10.1016/j.petrol.2015.01.012
Beg M, Singh P, Sharma S, Ojha U (2019) Shale inhibition by low-molecular-weight cationic polymer in water-based mud. J Petrol Explor Prod Technol 9:1995–2007. https://doi.org/10.1007/s13202-018-0592-7
Caenn R, Chillingar GV (1996) Drilling fluids: state of the art. J Petrol Sci Eng 14:221–230. https://doi.org/10.1016/0920-4105(95)00051-8
Chenevert ME, Sharma MM (2014) Maintaining shale stability by pore plugging. United State Patent 8,783,352. https://doi.org/10.26153/tsw/3511
Clark RK, Scheuerman RF, Rath H, Van Laar HG (1976) Polyacrylamide/potassium-chloride mud for drilling water-sensitive shales. J Petrol Technol 28:719–727. https://doi.org/10.2118/5514-PA
Dandekar AY (2013) Petroleum reservoir rock and fluid properties. CRC Press Boca Raton. https://doi.org/10.1201/b15255
Darley HC, Gray GR (1988) Composition and properties of drilling and completion fluids, 5th edn. Gulf Professional Publishing, Houston
Dehghani F, Kalantariasl A, Saboori R, Sabbaghi S, Peyvandi K (2019) Performance of carbonate calcium nanoparticles as filtration loss control agent of water-based drilling fluid SN. Appl Sci 1:1466. https://doi.org/10.1007/s42452-019-1446-8
Elmousalami HH, Elaskary M (2020) Drilling stuck pipe classification and mitigation in the Gulf of Suez oil fields using artificial intelligence. J Petrol Explor Prod Technol 10:2055–2068. https://doi.org/10.1007/s13202-020-00857-w
Fraser LJ, Harrington b, Albarrazin C, Snyder G, Donham F (2003) Use of mixed metal oxide fluid to combat losses in porous and fractured formations: two case histories. In: AADE 2003 national technology conference “practical solutions for drilling challenges”, Houston. https://www.aade.org/application/files/7515/7304/4596/AADE-03-NTCE-40-Fraser.pdf
Growcock FB, Patel AD (2011) The Revolution in non-aqueous drilling fluids. In: AADE national technical conference and exhibition, Houston. https://www.aade.org/application/files/9215/7261/8798/AADE-11-NTCE-33.pdf
Hossain ME, Al-Majed A (2015) Fundamentals of sustainable drilling engineering. Scrivener Publishing, Beverly. https://doi.org/10.1002/9781119100300
Hossain ME, Islam MR (2018) Problems related to the mud system. In: Drilling engineering problems and solutions: a field guide for engineers and students. John Wiley & Sons, pp. 77–137. https://doi.org/10.1002/9781118998632.ch3
Ismail AR, Aftab A, Ibupoto ZH, Zolkifile N (2016) The novel approach for the enhancement of rheological properties of water-based drilling fluids by using multi-walled carbon nanotube, nanosilica and glass beads. J Petrol Sci Eng 139:264–275. https://doi.org/10.1016/j.petrol.2016.01.036
Jung Y, Barry M, Lee J, Phuoc T, Soong Y, Martello D, Chyu M (2011) Effect of nanoparticle-additives on the rheological properties of clay-based fluids at high temperature and high pressure. In: AADE national technical conference and exhibition, Houston. https://www.aade.org/application/files/2515/7261/8809/AADE-11-NTCE-02.pdf
Kang Y, You L, Xu X, Liao Z (2012) Prevention of formation damage induced by mud lost in deep fractured tight gas reservoir in western sichuan basin. SPE-131323-PA 51:46–51. https://doi.org/10.2118/131323-PA
Lake LW (2007) Petroleum engineering handbook. Drilling engineering, vol 2. Society of Petroleum Engineers, Richardson
Mahmoud O, Nasr-El-Din HA, Vryzas Z, Kelessidis VC (2016) Nanoparticle-based drilling fluids for minimizing formation damage in HP/HT applications. In: SPE international conference and exhibition on formation damage control, Lafayette. https://doi.org/10.2118/178949-MS
Mahmoud O, Nasr-El-Din HA, Vryzas Z, Kelessidis V (2018) Effect of ferric oxide nanoparticles on the properties of filter cake formed by calcium bentonite-based drilling muds. SPE-184572-PA 33:363–376. https://doi.org/10.2118/184572-PA
McElfresh PM, Wood M, Ector D (2012) Stabilizing nano particle dispersions in high salinity, high temperature downhole environments. In: SPE international oilfield nanotechnology conference and exhibition, Noordwijk, Netherlands. https://doi.org/10.2118/154758-MS
Medhi S, Chowdhury S, Gupta DK, Mazumdar A (2019) An investigation on the effects of silica and copper oxide nanoparticles on rheological and fluid loss property of drilling fluids. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-019-0721-y
Mohammed AS (2017) Effect of temperature on the rheological properties with shear stress limit of iron oxide nanoparticle modified bentonite drilling muds. Egypt J Pet 26:791–802. https://doi.org/10.1016/j.ejpe.2016.10.018
Neff JM (2010) Fate and effects of water based drilling muds and cuttings in cold water environments. Shell Exploration and Production Company, Houston
Nelson PH (2009) Pore-throat sizes in sandstones, tight sandstones, and shales. Am Assoc Petrol Geol Bull 93:329–340. https://doi.org/10.1306/10240808059
Patel A, Stamatakis E, Young S, Friedheim J (2007) Advances in inhibitive water-based drilling fluids—can they replace oil-based muds? In: SPE international symposium on oilfield chemistry, society of petroleum engineers, Houston. https://doi.org/10.2118/106476-MS
Perween S, Beg M, Shankar R, Sharma S, Ranjan A (2018) Effect of zinc titanate nanoparticles on rheological and filtration properties of water based drilling fluids. J Petrol Sci Eng 170:844–857. https://doi.org/10.1016/j.petrol.2018.07.006
Vryzas Z, Mahmoud O, Nasr-El-Din HA, Kelessidis VC (2015) Development and testing of novel drilling fluids using Fe2O3 and SiO2 nanoparticles for enhanced drilling operations. In: International petroleum technology conference, Doha, pp. 6–9. https://doi.org/10.2523/IPTC-18381-MS
Vryzas Z, Zaspalis V, Nalbantian L, Mahmoud O, Nasr-El-Din HA, Kelessidis VC (2016) A comprehensive approach for the development of new magnetite nanoparticles giving smart drilling fluids with superior properties for HP/HT applications. In: International petroleum technology conference, Bangkok. https://doi.org/10.2523/IPTC-18731-MS
Zakaria M, Husein MM, Hareland G (2012) Novel nanoparticle-based drilling fluid with improved characteristics. In: SPE international oilfield nanotechnology conference and exhibition, Noordwijk, pp 12–14. Society of Petroleum Engineers. https://doi.org/10.2118/156992-MS
Zamani A, Bataee M, Hamdi Z, Khazforoush F (2018) Application of smart nano-WBM material for filtrate loss recovery in wellbores with tight spots problem: an empirical study. J Petrol Explor Prod Technol 9:669–674. https://doi.org/10.1007/s13202-018-0500-1
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, N., Alam, M.S. & Salam, M.A. Experimental analysis of drilling fluid prepared by mixing iron (III) oxide nanoparticles with a KCl–Glycol–PHPA polymer-based mud used in drilling operation. J Petrol Explor Prod Technol 10, 3389–3397 (2020). https://doi.org/10.1007/s13202-020-00933-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-00933-1