Abstract
The rechargeable Zn2+ ion batteries are promising for the sustainable energy storage device applications. Recently they have been extensively evaluated for finding new cathode material and prevention of dendrite growth at Zn plate anode. Herein we have evaluated redox active organic molecule 7,7,8,8Tetracyanoquino dimethane (TCNQ) as a cathode material for aqueous zinc battery with zinc plates as anode in 1 M ZnSO4. The charging/discharging of the battery was associated with formation and deformation of Zn-TCNQ complex, which was confirmed by XRD and FTIR. The specific capacity of cathode was found to be 123.2 mAh g−1 at 100 mA current density with 96% coulombic efficiency. Whereas specific capacity at 1 A current density was found to be 60 mAh g−1 with 94% coulombic efficiency. In a cycling experiments we observed the fading of capacity with time by partial dissolution of Zn-TCNQ complex. The fading of capacity was prevented by confining TCNQ molecules inside the nano structures of newly prepared covalent organic polymers. The confinement remarkably increased the capacitance to 171 mAh g−1 at 1 A current density. As the material is readily available and the absence of toxic inflammable volatile organic electrolytes in our battery this material offers a very good choice as cathode material for zinc battery.
Export citation and abstract BibTeX RIS
The increasing demand for efficient, environment friendly and safer materials for energy application is always a challenge in the contemporary world. Limited quantity of the energy resource, even though they are intermittent, and the remarkably increasing demand for the energy resource by the course of time is a matter of searching for novel materials. Thence such materials are always worthwhile to meet the requirement.1,2 Rechargeable batteries have obtained astonishingly large attention because of their extensive usage in portable and wearable devices such as pace makers and infusion devices like insulin pump for diabetics etc.3,4 And there is a serious concern about the safety and environment friendliness of the materials used in such batteries as cathode, anode, supporting electrolytes and the separator.5 Li ion batteries, in this regard, have been brought in to the attention of researchers long time before and they are incredibly dominated in the small portable electronic devices such as smartphones, laptops etc.6 However, Li batteries face serious safety problems mainly because of the inflammable toxic organic electrolytes used.7,8 Alternative, aqueous batteries are much safer to use because of environmental benignity, safe operation and low cost. Further, aqueous electrolytes have two order of magnitude higher ionic conductivity (∼1 S cm−1) than the non-aqueous electrolyte (∼0.001–0.01 S cm−1).9,10 There are multiple reports on aqueous batteries made up of alkali (e.g. Li+, Na+ and K+)9 and alkaline earth metals (e.g. Mg2+ and Ca2+).11 But always have the limitation of decaying cell efficiency and potential due to the formation of oxide and hydroxides.12 There are also reports on the use of multivalent metal ion like Al3+ and Zn2+ aqueous rechargeable batteries.13 The multivalent ions battery have higher specific capacities and energy densities because of involvement of multiple ions in redox reactions. Aluminum-ion batteries have volumetric and gravimetric capacities of 8040 mAh cm−3) and 2980 mAh g−1, respectively, of the aluminum anode. But the formation of aluminum oxide film on anode surface in aqueous electrolyte limits the cell efficiency and cell potential.
On other hand Zinc ion battery, which has been explored over the century with metallic Zinc anode regarded as the one of the safest electrode material in aqueous batteries due to its low redox potential (0.76 V vs SHE), high theoretical capacity (5845 mAh cm−3, 819 mAh g−1), and good compatibility with water.14,15 Its abundance in the Earth's crust, nontoxicity, and stability in air further benefits its sustainability and large-scale production. These properties renaissance the recent developments in the rechargeable Zn2+ ion batteries.9 The use of neutral or slightly acidic solutions to replace alkaline electrolytes can suppress the dendritic growth of the Zn anode. Whereas suitable cathode material can galvanize rechargeable zinc battery. Significant work has been reported with Zn anode and organic redox molecules or polymers as a cathode for sustainable development. Calix[4]quinone (C4Q) cathode based rechargeable metal zinc batteries showed high capacity of 335 mAh g−1 with an energy efficiency of 93% at 20 mA g−1.16 The tetrachloro-1,4-benzoquinone (p-chloranil) cathode delivered high specific capacity of ≥200 mAh g−1 and attractive specific energy of >200 Wh kg−1 with an energy efficiency of ∼95%.17 The pyrene-4,5,9,10-tetraone (PTO) cathode reported with high specific capacity of 336 mAh g−1 and PTO//Zn full cell reported a high energy density (186.7 Wh kg−1).18 PANI cathode reported with a high capacity of 180 mAh g−1.19
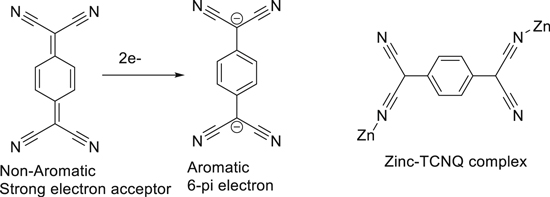
Here we are reporting 7,7,8,8-Tetracyanoquinodimethane (TCNQ) as a new potential cathode material for rechargeable aqueous Zinc ion battery. TCNQ is an excellent electron acceptor, extensively used as electron acceptor for organic semiconductors. Apart from having a very strong electron withdrawing groups on TCNQ, its inherent ability to accept electrons and consequent formation of the stable aromatic ring so as to satisfy the Huckel rule of aromaticity is the reason of its outstanding electron accepting capability Fig. 1.20
Figure 1. TCNQ stability with Huckel aromaticity.
Download figure:
Standard image High-resolution imageHence we have explored fabrication and evaluation of a rechargeable zinc battery with TCNQ cathode and aqueous ZnSO4 as electrolyte.21,22 The stability was increased by confining it inside the nanoporous covalent organic material. ZnSO4 is considerably much better electrolyte for multivalent ion-intercalation batteries compared to non-aqueous electrolytes. Additionally, the use of aqueous medium is remarkably a judicious choice for the sake of user and environment friendliness.
Experimental
Materials
7,7,8,8-Tetracyanoquinodimethane (TCNQ), Nafion® (20%in hexane), ZnSO4, Cyanuric chloride (CC), pyrene, methane sulfonic acid were purchased from sigma Aldrich and multi walled carbon nanotubes (MWCNT) was purchased from SRL pvt. Ltd. and have been used without further purification. Zinc metal plates were purchased from research-lab fine chem industries Mumbai. It was cleaned with sulphuric acid and acetone. Carbon paper was purchased from spectrum chemicals. Before use it was made hydrophilic by treatment of concentrated nitric acid as reported elsewhere.23
Synthesis of CCP
To 6.58 mmol (1.214 g, 1.33 equivalent) of CC in 30 ml 1,2-Dichloroethane 4.95 mmol pyrene (1 g, 1equivalent) was added, stirred for 5 min to get a homogenous solution. Methane sulfonic acid was added (3.32 ml, 7equivalent) to the stirring solution and heated to reflux at 80 oC for 6 h. After 1 h of the reaction the color was changed to black solution and solid formation started, the reaction was allowed continuously for 6 hours for completion of the reaction. The reaction mixture was kept for cooling and washed with methanol, acetone and distilled water several times, filtered and boiled the solid in methanol and acetone separately, filtered, dried and stored as black powder and denoted as CCP (94.8% yield).
Preparation of electrode
The electrode was made by dip coating hydrophilic carbon paper with a paste consisting of TCNQ, CNT, Nafion, having 60:30:10 weight ratio. Before making the paste, TCNQ and CNT were ground in to fine powder in a clean mortar paste. The thoroughly ground mixture was dispersed into an isopropanol and sonicated for 45 min. It followed by the addition of Nafion® solution and ball milled to get the homogeneous dispersion. The so obtained dispersion was dip coated on the carbon paper and used in experimental studies. The TCNQ confinement inside the CCP i.e. TCNQCCP was prepared by dissolving 140 mg TCNQ in acetone followed by 70 mg CCP. The solution was mixed thoroughly and allowed to evaporate at 40 °C. After complete evaporation acetone was added again and evaporated, repeated for five times and used for the electrode preparation.
General physical characterization
FTIR spectra were recorded with a Perkin-Elmer (Germany) GXFTIR system through the 400–4000 cm−1 range. The XRD diffraction patterns were recorded with a PAN Analytical (Germany) system using the CuKα line (λ = 1.5406 Å) through 5 to 60° range at a scan rate of 10° min−1. The surface morphology was visualized using a JSM-7100F, Japan field emission scanning electron microscope. The surface area was analysed by Micromeritics BET surface area analyser with N2 adsorption/desorption experiment at 150 °C. Raman spectroscopy (Model: Alpha, Make: WITec, Germany) was performed in the 400−3000 cm−1 frequency range with a 514 nm laser source. Electrochemical studies were carried out using CHI 760E Electrochemical Analyzer (CH Instruments, USA) in 1 M ZnSO4 solution at room temperature.
Result and Discussion
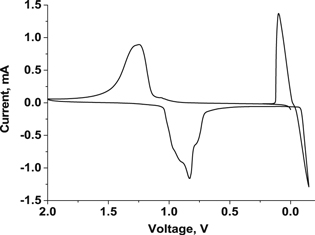
7,7,8,8Tetracyanoquinodimethane (TCNQ) is the organic compound with excellent electron accepting ability widely used for the formation of charge transfer salt in molecular electronics.24 It has been reported that controlled formation of TCNQ-Zn complex in aqueous medium indicating reversible coordination and de-coordination of the Zn2+ ions.25 Figure 2 shows the recorded cyclic voltammogram of reversible coordination and de-coordination of the Zn2+ ion with TCNQ paste coated carbon paper electrode in the potential window of −0.2–2.0 V using 1 M ZnSO4 aqueous electrolyte. The voltammogram was recorded versus Zinc plate at 50 mV s−1 scan rate. In the given potential window, TCNQ was found to be excellently electrochemically active with symmetrical redox wave. The excellent redox wave is due to the permselective motion of a hydrated counter ion into and out of TCNQ layer on carbon paper substrate concomitant with the electron Motion.26,27 Classically TCNQ shows one redox peak associated with single electro transfer in aqueous medium.27 Similarly, the obtained voltammogram showed oxidation peak at 1.4 V whereas reduction peak at 0.65 V. It is due to the coordination/de-coordination of Zn2+ ion with TCNQ molecules. The separation between oxidation and reduction peaks was ∼0.8 V indicating high ohmic resistance. The nature of the peak indicates that there is very stable two states of the TCNQ, one is the reduced and other is the Zn coordinated complex accord with the following equation.
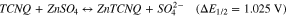
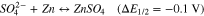
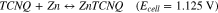
These are similar with the zinc intercalation mechanism reported in the earlier study15,28–30 with maximum operating voltage of 1.125 V. It is well below the thermodynamic potential of water oxidation, hence neither O2 nor H2 could evolve on respective electrodes.
Figure 2. Cycle voltammogram of the TCNQ electrode with Zn plate as reference and coulter electrode in 1M ZnSO4 solution, scan rate 50 mV s−1.
Download figure:
Standard image High-resolution imageThe formation of Zn-TCNQ complex during cycling of the electrode was confirmed by recording XRD and FTIR spectra. The recorded XRD was shown in Fig. 3A. Compared to the neat electrode before cycling experiment there is a clear peak shift at 19.80° and the peak intensity was significantly diminished at 22.75° in addition to that there are peaks appeared at 8.90°, 12.96° and 15.88° in the electrode after the cycling experiment. In order to confirm the additional peaks appeared, a Zn-TCNQ complex was prepared as reported procedure31 and matched their XRD pattern with the electrode after cycling experiment. There was exact matching with the pattern as shown in the Fig. 3A, indicating the formation of Zn-TCNQ complex during cycling. FTIR spectra of the electrode before and after the cycling were taken and analyzed as follows (Fig. 3B). The band at 2226 cm−1 for pure TCNQ was shifted to 2134 cm−1 which was identified for CN stretching frequency of [TCNQ]− ion. The presence of sharp peak at 1507 cm−1 is further evidence for reduced TCNQ, C=C stretching vibration in the ring. The peak at 821 cm−1 indicates for the C-H stretching in the [TCNQ]−.32 These analyses clearly confirm the formation of Zn-TCNQ complex during cycling of the electrode.
Figure 3. XRD and TFIR spectra of the TCNQ electrode recorded before and after cycling (A) XRD and (B) FTIR.
Download figure:
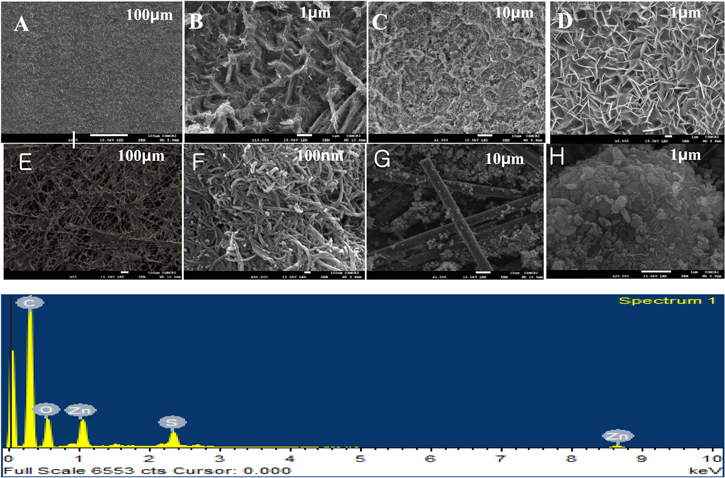
Standard image High-resolution imageFigure 4 shows the SEM analysis of Zn plate, anode before and after cycling. Before cycling there was no dendrite formation on the surface of the anode (Figs. 4A & 4B). But after cycling, there is clear formation of dendrite or plate type structure due to stripping and plating of Zn. The formation of dendrite was uniform throughout the anode (Figs. 4C & 4D). These observations are similar to the earlier reports.9,15 On performing the charge, the zinc anode is oxidized to Zn2+, which passes across the electrolyte and is stored by TCNQ at the cathode through a Zn-TCNQ coordination reaction. The TCNQ gets reduced by the two electrons generated and forms [TCNQ]−. As soon as the formation of the reduced species of TCNQ at the cathode surface, Zn2+ forms a coordination complex and the mechanism has been predicted (Fig. 5). It was confirmed by XRD and FTIR (Fig. 2).
Figure 4. FESEM images of Zinc plate anode surface before (A) & (B) and after (C) & (D) the cycling experiment. FESEM images of cathode surface before (E) & (F) and after (G) & (H) the cycling experiment. Elemental mapping of the cathode surface after cycling showing presence of Zinc ion (I).
Download figure:
Standard image High-resolution imageFigure 5. Possible mechanism and the cell reaction.
Download figure:
Standard image High-resolution imageThe formation of Zn-TCNQ complex was also supported by SEM analysis. The images were presented in Figs. 4E–4H. Before cycling we could observe the uniform coated carbon paper surface with evidence of presence of carbon nanotubes (Fig. 4F). But after cycling we could observe the formation of aggregates of different size and shapes (Figs. 4G & 4H). EDX mapping of aggregates shows the presence of Zn, nitrogen and carbon confirming Zn-TCNQ complex formation (Fig. 4I).
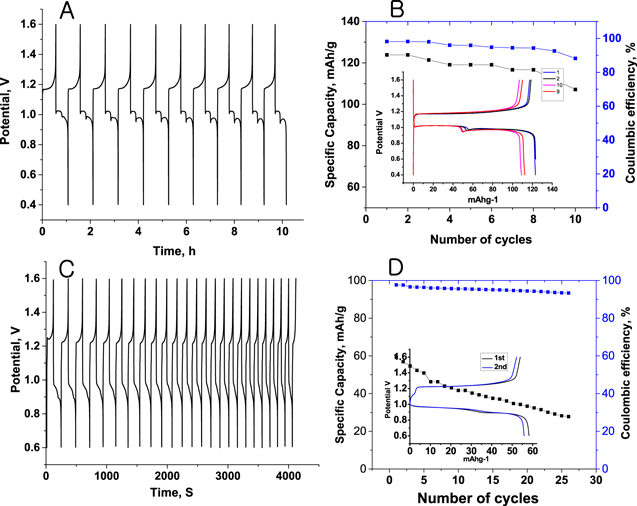
To evaluate the usefulness of TCNQ as a cathode material for rechargeable Zn-ion battery, galvanostatic charging/discharging was performed in 1 M ZnSO4 solution in 0.4–1.6 V potential window. The obtained charging/discharging graphs were presented in Fig. 5. The specific capacity was found to be 123.2 mAh g−1 at current density 100 mA g−1 with corresponding coulombic efficiency of ∼96% in an initial few cycles as shown in Figs. 6A and 6B. Theoretical specific capacity was calculated as 262.7 mAh g−1 according to the equation Q = nF/3600*MW; Where n, F, and MW are number of electrons involved, Faraday's constant and molecular mass respectively. After 8 cycles there was steady fading in capacity as well as coulombic efficiency due to dissolution of Zn-TCNQ complex at cathode and dendrite formation at Zn plate anode.
Figure 6. Charging/discharging of TCNQ cathode at 100 mA current density (A), corresponding specific Capacity and coulombic efficiency (B). Charging/discharging at 1A current density (C), and corresponding specific capacity and coulombic efficiency (D).
Download figure:
Standard image High-resolution imageTo avoid the dissolution Zn-TCNQ complex at low current density, charging/discharging were performed at high current density of 1 A g−1. Though at high current density, there is fast expansion and contraction of active material, which deteriorate the performance. The charging/discharging data are presented in Fig. 6C with corresponding specific capacity and coulombic efficiency in Fig. 6D (The potential versus specific capacitance graphs were given in set 6B & 6D). The obtained initial specific capacity was 60 mAh g−1 with corresponding coulombic efficiency of ∼94%. In a 50 cycles there was 50% reduction in the specific capacity indicating poor stability of the electrode. The low specific capacity at high current density is due to the sluggish transport of Zn2+ ion into the TCNQ matrix or interfacial resistance. In order to improve the cycling performance of the TCNQ cathode, the experiments were performed with coating thin layer of Nafion® on the active electrode surface by dip coating. The Nafion is used because of its Zn2+ ion permeability. The cycling data are presented in Fig. 7. The specific capacity of the electrode was found to be 110 mAh g−1 at 1 A current density. It is higher than the specific capacity of the electrode without Nafion® coating. The high capacity is due to the permselectivity of Nafion® to Zn2+ ions. It partially prevents leaching of Zn-TCNQ complex into the electrolyte but transports Zn2+ ions during charging/discharging process. But coating of Nafion® has insignificant effect on its stability.
Figure 7. Charging/discharging of Nafion® coated carbon electrode at 1 A current density (A), and corresponding specific capacity and coulombic efficiency (B).
Download figure:
Standard image High-resolution imageThe continuous fading of the specific capacity is due to sparingly solubility of Zn-TCNQ complex. This continuous dissolution of the material lead to the active material loss causing fading the overall specific capacity of the battery.
The specific capacity of the TCNQ cathode was compared with literature known organic cathode material for Zn2+ ion batteries. These data were comparable with the data reported by Trinidad et al. for electrochemically polymerized polyaniline as cathode in ZnCl2-NH4Cl electrolyte. They reported the specific capacity of 30.4 mAh at 1 mA and it was decreasing drastically to ∼54% while 10 times increasing in the charging current density.33 Shengqi Li et al. extended this study by changing the conditions of electropolymerisation. They performed the PANI synthesis electrochemically in 1-ethyl-3-methylimidazolium-ethyl sulfate (EMIES), an ionic liquid to restrict the polymerization due to its excellent viscosity in H2SO4.34 The capacitance of this material was remarkably increased to 128 Ah kg−1 with coulombic efficiency of 97%.
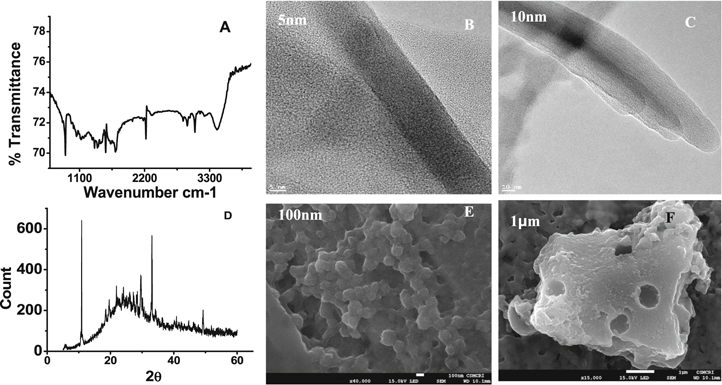
TCNQ is a small organic molecule with very low water solubility. However, during the prolonged charging discharging experiment the molecule may be losing its electrode stability leading towards considerable capacitance decline. Kundu et al. reported similar small size organic molecule as cathode material for aqueous zinc battery.17 They could remarkably improve the electrochemical cycling by confining the small molecule inside the nano channels of the specially designed mesoporous carbon material. In order to solve the probable chance of dissolution of the material the molecules have been entangled in a specially prepared triazine based covalent organic framework, CCP. Several materials have been introduced as host materials to trap the small molecules either chemically or physically to prevent their dissolution. Mesoporous carbon,35 carbon nanotubes,18 hierarchical porous carbons,36 porous organic polymers,37 metal organic frame works(MOFs)38 and covalent organic frame works (COFs)39 are some of the examples of such materials. MOFs and COFs have obtained very special attention because of the possibility of engineering their pore size very precisely by judicious choice of synthetic methods and starting precursors. MOFs have metal centers with various coordination sites which offer more topologies, whereas COFs lack of such heavy metal centers that can offer low densities with comparatively good thermal and chemical stability. More over COFs consist of light elements as very strong covalent linkers such as N, S and O atoms to accommodate the guest molecules.40,41 The newly synthesized covalent organic frame work, CCP has incredible structural properties such as specific pore size, structural robustness and insoluble in almost all solvents. We have synthesized a novel nitrogen rich CCP and modified our electrode material as TCNQCCP. The synthetic procedure was given as follows. Figure 9A. To 6.58 mmol (1.214 g, 1.33 equivalent) of TCT in 30 ml 1,2-Dichloroethane 4.95 mmol pyrene (1 g, 1equivalent) was added, stirred for 5 min to get a homogenous solution. Methane sulfonic acid was added (3.32 ml, 7equivalent) to the stirring solution and heated to reflux at 80 °C for 6 h. After 1 h of the reaction the color was changed to black solution and solid formation started, the reaction was allowed continuously for 6 h for completion of the reaction. The reaction mixture was kept for cooling and washed with methanol, acetone and distilled water several times, filtered, dried and stored as black powder and denoted as CCP (94.8% yield). Figure 8A shows the FTIR spectra of CCP as well as the starting precursors; cyanuric chloride (CC) and pyrene (p). The formation of CCP was followed by absence of strong stretching frequencies at 854 cm−1 for C–Cl bonds. The presence of other stretching frequencies at 1400 cm−1 C=C bond, 1577 cm−1 C=N bond, 833 cm−1 for C–H bond, 1063 cm−1 for C–N bond confirm its formation. The Fig. 8D, the Raman spectra of CCP confirm its formation due to the presence of vibration at 1351 cm−1 for C–N bond and 1593 cm−1 for C=C bond.
Figure 8. (A) FTIR spectra of CCP. (B) powder XRD of CCP with starting precursors. (C) BET surface area of CCP, differential pore volume versus pore width graph in set. (D) Raman spectra of CCP. E&F). SEM images. G&H) TEM images of CCP.
Download figure:
Standard image High-resolution imageThe XRD pattern of the carbonaceous N-doped material, CCP derived from covalent triazine framework is shown in Fig. 8B. It is seen that the synthesized material exhibits two broad diffraction peaks which are similar to typical graphene stacks with a low degree of graphitization. The observed peak at ∼2θ = 42.57°, corresponds to (100) crystallographic plane reflection obtained from honeycomb like sp2 hybridized carbon. Similarly, peak observed at around 21.26° refers to (002) crystallographic planes which arises from consistent and parallel stacking domains of graphene like sheets. The above peaks observed form the XRD confirms that the synthesized material partially maintains the graphitic behavior. The interlayer distance was calculated as 0.441 nm for CCP which was higher than that in the typical graphite.42 The XRD pattern of the reactants were also shown along with the product for the sake of comparison and it was interesting to note that the product was mesoporous amorphous in nature whereas.
The reacting precursors were found to be perfectly crystalline. The porosity of the material was measured by BET isotherm (Fig. 8C). The surface area was found to be 5 cm3 g−1. The surface morphology of the material was observed by SEM (Figs. 8E & 8F) and TEM images (Figs. 8G & 8H). The porosity of ∼1 nm is visible from TEM.
TCNQCCP was prepared by dissolving 140 mg TCNQ in acetone followed by 70 mg CCP. The solution was mixed thoroughly and allowed to evaporate at 40 °C. After complete evaporation acetone was added again and evaporated, repeated for five times. The dried powder thus obtained was used as electrode active material with CNT and Nafion® in the 60:30:10 ratios, mixed, ground in mortar pestle and made slurry in isopropanol. Schematically represented in the Fig. 9B. Confinement of the TCNQ in the CCP was analysed by XRD and FTIR analysis Figs. A & D. The characteristic peaks for TCNQ and CCP were observed in both analyses. SEM (Figs. E & F) and TEM (Figs. B & C) images further confirm the confinement of the material (Fig. 10).
Figure 9. (A). Reaction scheme for laboratory synthesis of CCP. (B). Schematic representation of electrode preparation.
Download figure:
Standard image High-resolution imageFigure 10. FTIR (A), TEM (B) & (C), XRD (D) and SEM images (E&F) of TCNQ after confinement in CCP.
Download figure:
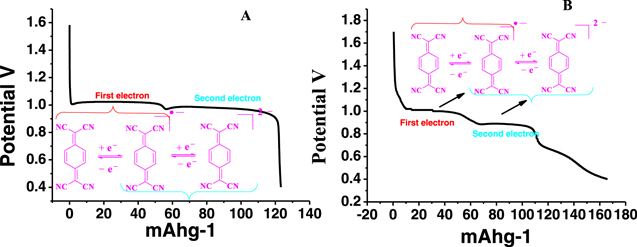
Standard image High-resolution imageInitially we performed the cyclic voltammogram for 100 cycles in order to check the electrode stability of the material as well as the regaining of the redox active behavior after prolonged usage. Interestingly the redox peak declining was found to be insignificant after 100 cycling and no potential shift was found even though there is slight difference in the CV compared to the simple TCNQ(Fig. 11). The presence of two distinctive reduction peaks is observed with one prominent oxidation peaks and small hump in TCNQ immobilized CCP. Whereas neat TCNQ showed the one prominent oxidation/reduction peak and small humps. This difference may be due to the interaction of TCNQ with CCP host material which favors second oxidation/reduction.
Figure 11. Charging/discharging of TCNQCCP coated carbon electrode at 1 A current density (A) and corresponding specific capacity for few selected first and last cycles Charging/discharging of TCNQCCP coated carbon electrode at 1 A current density (B). Cyclic voltammogram first and last of 100 continuous cycles was also shown (C).
Download figure:
Standard image High-resolution imageTwo redox reactions are identified for TCNQ involving two consecutive redox reactions through a radical anion. The characteristic two step discharge profile can be therefore matched with this redox mechanism of TCNQ. However, the discharge profile for TCNQ immobilized CCP has an additional hump which may be due to the host-guest interaction. Figs. 12A & 12B.
Figure 12. (A). Characteristic two step discharge profile corresponding to the first and second redox reactions of TCNQ and (B). CCPTCNQ.
Download figure:
Standard image High-resolution imageThe stability is presumably due to the TCNQ entanglement in to the porous structures of the CCP where the large number of nitrogens from CCP can facilitate the guest molecule to accommodate irreversibly. The presence of highly electron withdrawing groups, cyanides make TCNQ as an electron deficient molecule by negative inductive effect or +I effect. Hence the electron affinity of the molecule will be very high (as π-acid) and consequently can make π-π stacking interaction with the electron surplus species having hetero atoms containing molecules such as N, O, S.43 Moreover, the porous nature of the CCP can help in shuttling of the zinc ions which may also improve the cell performance as well as the electrode stability. The pore size of CCP was found to be 21–29 Å (Fig. 8D) and supported by the SEM and TEM images (Fig. 8).
The presence of nanochannels make the TCNQ to be confined and the nitrogen atoms in CCP facilitate accommodating the incoming molecule inside porous structure by providing weak interaction such as π-( interaction. These nanostructures further help the hydrated zinc ion enter to be coordinated with the organic molecule reversibly while charging discharging process. The cathode surface was analysed before and after discharge experiment by XRD and FTIR (Figs. 13A & 13B). Characteristic peaks were observed 24°–31° for TCNQ which were silent after discharge and new peaks appeared 31°–39° in the XRD pattern. Similarly, characteristic vibrations were silent in the FTIR spectrum after discharging and characteristic peak was observed at 2135 cm−1 for CN stretching frequency of [TCNQ]- ion. The peak at 821 cm−1 indicates the C–H stretching in the [TCNQ]−. SEM images of both cathode and anode surfaces were seen after 100 cycles and EDX mapping of the cathode confirms the zinc ion on the cathode electrode Fig. 13 (anode for D, cathode for C).
Figure 13. (A) XRD. (B) FTIR. (C) & (D) SEM images of cathode and anode respectively. EDX color mapping images for elements C,N and Zn of cathode after cycling also shown.
Download figure:
Standard image High-resolution imageThe galvanostatic cycling experiment was performed for this and it was found that the discharge capacitance was remarkably increased to 171 mAh g−1 and decreased to 134.3 mAh g−1 after 100 cycles at 1 A current density (Fig. 11). Interestingly the coulombic efficiency was found to be higher than 100%. And it may be due to the contribution from air oxidation at TCNQCCP electrode. Having found the ability of the TCNQ being used as cathode material we have fabricated a battery with our material as cathode and zinc plate as anode with a glass membrane separator in ZnSO4 electrolyte. Fabricated battery and its working with a LED bulb was shown in Fig. 14. Zinc ions can pass through the membrane towards cathode to form a TCNQ− complex and the electrons generated move through the external circuit. The open circuit potential was found to be 1.1 V. The chemical modification of TCNQ is a promising way to improve the electrode stability that can either be post-polymerized or chemically attached to the stable polymeric backbone which we are currently working on.
Figure 14. Working of LED with Fabricated aqueous zinc battery. (1) Zinc metal as anode, (2). Silica glass membrane separator, (3). Coated carbon paper as cathode.
Download figure:
Standard image High-resolution imageConclusions
7,7,8,8-Tetracyanoquinodimethane (TCNQ) was used as cathode material for aqueous zinc battery. The capacity was comparatively better to the reported organic material like polyaniline, polypyrrole and polythiophene. It was found to be 123.2 mAh g−1 at 100 mA current density with coulombic efficiency of 94%. The stability of the cathode was increased by confining TCNQ inside the specially designed organic porous polymer, CCP which was confirmed by XRD, FTIR, TEM and BET analysis. The confinement not only increased the stability but also boost the capacity. The initial capacity was 171 mAh g−1, much higher than that without confinement. With cycling the capacity was decreased to 134.3 mAh g−1 after 100 cycles at 1 A current density. The results are promising for the TCNQ material to be used as cathode in aqueous zinc battery. Readily availability, inherent chemical stability and very limited dissolution in to the electrolyte during the experiment make this material further very suitable for the application in aqueous zinc battery.
Acknowledgments
Director CSIR-CSMCRI is acknowledged for continuous support and encouragement. Instrumentation facility provided by Analytical Discipline & Centralized Instrument Facility, CSIR-CSMCRI, Bhavnagar, are gratefully acknowledged. RKN thanks for the financial support (grant number EMR/2016/00l977) from Science and Engineering Research Board, DST India. CSIR-CSMCRI manuscript number– 085/2019.