Abstract
As genetics becomes increasingly integrated into all areas of health care and the use of complex genetic tests continues to grow, the clinical genetics workforce will likely face greatly increased demand for its services. To inform strategic planning by health-care systems to prepare to meet this future demand, we performed a scoping review of the genetics workforce in high-income countries, summarizing all available evidence on its composition and capacity published between 2010 and 2019. Five databases (MEDLINE, Embase, PAIS, CINAHL, and Web of Science) and gray literature sources were searched, resulting in 162 unique studies being included in the review. The evidence presented includes the composition and size of the workforce, the scope of practice for genetics and nongenetics specialists, the time required to perform genetics-related tasks, case loads of genetics providers, and opportunities to increase efficiency and capacity. Our results indicate that there is currently a shortage of genetics providers and that there is a lack of consensus about the appropriate boundaries between the scopes of practice for genetics and nongenetics providers. Moreover, the results point to strategies that may be used to increase productivity and efficiency, including alternative service delivery models, streamlining processes, and the automation of tasks.
Similar content being viewed by others
INTRODUCTION
The utilization of genetic testing in clinical settings has greatly increased over the past 10 years,1,2 with one study projecting annual growth in genetic test use of 23% between 2014 and 2024.3 This trend has been driven in part by the rapid decline in the cost of sequencing4 and has been accompanied by the advent of clinical genome-wide sequencing (GWS; including exome and genome sequencing).5 As a result, demand for counseling and consultations with clinical genetics professionals has also grown rapidly, resulting in concerns about potential workforce shortages and insufficient health system capacity to meet this growing demand.6,7,8 Moreover, continued growth in the clinical implementation of GWS is likely to put further pressure on the clinical genetics workforce because GWS requires more intensive decisional support for both patients and health-care practitioners than for less comprehensive genetic tests. This is due to the possibility of secondary findings, data storage and privacy concerns, difficulty in interpreting test results, and the need to support patients who must deal with the complex, and often unanticipated, psychological and informational impacts of genomic testing.9 Indeed, it is unclear how the genetics workforce will be able to meet the growing demand for GWS testing, given that the literature suggests that there is already a shortage of clinical geneticists (CGs; i.e., physicians with a board-certified specialization in medical genetics) and genetic counselors (GCs). For example, a substantial number of CG residency openings go unfilled each year,10 and it has been estimated that there are only 7000 GCs worldwide.11
Understanding the current composition and capacity of the clinical genetics workforce is a prerequisite for effective strategic planning by health-care systems in light of the expected growth in demand for genetics services over the next 10–20 years. As such, our objective for this scoping review is to summarize the available evidence on the current state of the genetics workforce, focusing in particular on the number and types of professionals involved, their ability to deliver genetics services, and opportunities for increased efficiency through task-sharing, delegation, alternative service delivery models, and augmentation of services through the use of technology. Previous reviews have assessed present and future characteristics of the GC workforce,12,13 alternative service delivery models,14 genetics education content,15 and attitudes of health-care providers about their perceived roles in genetics.16 However, these studies have tended to focus on a single indication or setting, which is suboptimal given the ability of clinical genetics professionals to practice in all clinical areas and the high level of international labor mobility for genetics professionals in regions like North America. As a result, our review aims to compile the available evidence about the composition and capacity of the clinical genetics workforce across all high-income countries and regions, with the goals of better understanding the global labor market for genetic health-care professionals and of identifying possible policy solutions to labor shortages that could be applied in multiple jurisdictions.
MATERIALS AND METHODS
This review was conducted according to the Arksey and O’Malley methodological framework for scoping reviews,17 along with recommendations from the Joanna Briggs Institute18 and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.19 A full description of our methods appears in the Supplemental Appendix.
Search strategy
We searched five databases (MEDLINE, Embase, CINAHL, PAIS, and Web of Science) for articles published between January 2010 and April 2019. Gray literature publications were identified from sources listed in the Canadian Agency for Drugs and Technologies in Health’s Gray Matters Checklist and from relevant professional organizations related to the genetics workforce.20 In addition, new publications identified through a PubMed alert for related publications were included until the end of primary data extraction (30 July 2019), and reference mining was used to identify additional studies.
Study selection
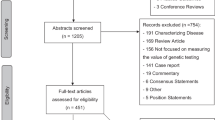
Publications in English, French, or Spanish that described the genetics workforce in high-income countries (as listed in the Supplemental Appendix)21 were retained. Relevant characteristics included the number and type of genetics professionals, scope of practice, time needed for tasks, legal recognition, wait times, case loads, referral patterns, professional issues, impacts of technology, and compensation structure. Nonempirical papers and professional practice and clinical evaluation guidelines were excluded. Title and abstract screening and full-text review were all performed by two independent reviewers (N.K., K.B.), with any disagreements resolved by a third reviewer (N.D.). Potentially relevant studies identified through citation mining and during the course of searching for gray literature were evaluated for inclusion based on the same criteria. The reasons for exclusion of database search records are reported in the PRISMA diagram (Fig. 1).
Data extraction
Data extraction took place in two phases. Primary data extraction was conducted by one of two coders and a common set of data points were extracted for all studies, including basic study characteristics, data sources and methods, health-care professional data, and factors influencing workforce supply and demand. The results were grouped according to three main themes: (1) number and type of individuals in the workforce, (2) scope of practice, and (3) interventions that increase capacity. The results were synthesized within their groups, and secondary data extraction was conducted as necessary on subsets of studies to extract data on specific themes of interest identified during the course of analyzing the results of the primary data extraction.
RESULTS
Full-text review was performed on 304 publications from the database search, of which 121 were included in the review (Fig. 1). Twenty-six gray literature documents, and 23 additional peer-reviewed studies found through citation mining and during gray literature search were also included after full-text review. In total we included 170 records reporting on 162 unique studies (Supplementary Table A). The majority of included studies focused on the North American (101/162, 62%) or European (32/162, 20%) workforces (Table 1). In addition, 69% (111/162) reported on genetics providers and 48% (78/162) discussed nongenetics providers.
For the purposes of presenting thematic results in this review, we created a conceptual model of the genetics workforce outlined in Fig. 2, which divides the workforce into genetics specialists and other health-care providers and defines capacity as the collective ability of these two groups to perform the tasks involved in delivering clinical genetics services. The key drivers of capacity that emerged from our results were (1) the type and number of genetics specialists, (2) their scopes of practice, (3) time spent on genetics tasks, (4) case loads, (5) the scope of practice for nongenetics specialists who provide genetics services, and (6) opportunities to increase genetics services capacity.
Type and number of genetics specialists
The number of full-time equivalent (FTE) providers per 100,000 inhabitants is a commonly used metric in health-care planning. Although there was no agreement in the literature about what the ideal ratios would be to provide adequate genetics services, the number of GCs available to meet clinical demand in several jurisdictions (the United States, Europe, Chile, and Australia) was estimated as between 0.2 and 1.2 FTEs per 100,000 inhabitants,22,23,24,25,26,27,28,29 and five of these studies reported a shortage of GCs based on these ratios.22,23,24,25,29 Workforce surveys conducted between 2016 and 2019 indicated that there were approximately 4900 GCs in the United States and Canada, of whom over 400 work in Canada.30,31,32 The number of students enrolled in genetic counseling programs in North America has increased by 40% since 2012.33,34 As of 2017, there were 220 GCs working in clinical roles in Australia, though 677 individuals held an Australian genetic counseling degree.28,35 In 2012, it was estimated that there were 494 GCs and 122 genetic nurses in Europe.27 The regulatory framework for GCs and genetic nurses was highly variable across jurisdictions, and a number of publications discussed different elements of legal regulation, professional recognition, registration, and licensure.3,27,35,36,37,38,39
Provider–population ratios for CGs were estimated as 0.3 FTE per 100,000 inhabitants in Chile and 0.6 FTE per 100,000 inhabitants in Australia, and it was argued that these ratios indicated a shortage.22,23,28 Included studies reported the absolute number of CGs in Portugal (30 practicing), Chile (28 practicing), the United States (over 250 practicing survey respondents), and Australia (approximately 150 medical genetics fellowship graduates).28,35,40 Approximately five new CGs graduate in Australia per year, and it was estimated that in the next 15 years, 25% of Australian CGs will retire.28 Three publications about North American training programs reported that about half of medical genetics residency spots remain unfilled each year,10,41,42 and there were also vacancies in genetics pathologist residency programs.43 In addition, up to half of employment positions for CGs were vacant in the United Kingdom and the United States.44,45,46
There were fewer publications of this type about the laboratory workforce. According to two surveys, there were approximately 300 clinical laboratory geneticists (CLGs) in Europe. The CLG title is available in 60% of European countries, and although the educational pathway and scope of practice depends on the subspecialty and country, this position is usually filled by a nonmedical doctor who holds a PhD in genetics and/or has other specialized training.47,48 Similar roles exist in the United States and Canada (with varying specializations and workforce challenges), but no studies reporting on the CLG workforce in North America were found. In 2017, there were 51 senior genetics pathologists in Australia.35 Four publications described laboratory staff in Canada and the United States, finding that only a small proportion of individuals (1–5%) were recognizable as being specialized in genetics.49,50,51,52 Two workforce surveys of genetic laboratory scientists in the UK National Health Service showed that the largest employee groups were clinical scientists and genetic technologists/practitioners (39.7% and 31.5% of workforce in 2016); and there was a small group of bioinformaticians employed (30 in 2016).53,54 Workforce data showed the total number of staff increased by 41% over the prior 6 years while the FTE equivalent increased by 39%.54
Scope of practice for genetics health-care providers
A number of studies attempted to delineate the perceived scope of practice of GCs and CGs. Table 2 summarizes genetics-related tasks. Overall, it was agreed that most tasks could be done by either type of provider. Taking family histories, risk assessment, patient education, and psychological assessment and support were considered appropriate for GCs by both GCs and CGs,16,40,55,56 whereas medical examination, management of complex cases, and making diagnoses were deemed to fall within the exclusive purview of CGs.16,26,55,56,57,58 Administrative tasks such as initial patient contact,26,37 appointment logistics, handling testing samples, and billing were frequently performed by GCs.35,56 Whether a GC took on tasks traditionally performed by a CG was correlated with years of experience and professional relationship with the CG,57,59 rather than more training or education.57 In a European study, 74% of GCs ordered genetic tests at least sometimes,37 and in an Australian study looking at genomics tasks, 26.2% of GCs and 85.7% of CGs ordered genome or exome sequencing.35 In contrast, in some countries, the scope of practice for GCs faces strict legal constraints—for example, in Austria genetic counseling can only be delivered by a physician.60 When nurses work as genetic nurses, the scope of practice appears to be analogous to GCs. Five studies discussed the roles of nurses as specialists providing genetic counseling, one of which also described the role of midwives.27,37,61,62,63
Frequently cited clinical specialties for GCs in direct patient care were cancer, prenatal care, general genetics, and pediatrics.25,35,64 As well, areas of specialization for GCs involved in direct patient care were reported in private practice settings,65 pharmacogenomics,66 and public health.67 A growing number of GCs take on roles beyond the provision of direct patient care, with the proportion of GCs in the United States working in direct patient care having decreased from 65% in 2016 to 59% in 2019. The most common employment classifications for GCs working in nondirect patient care were industry, education, research, and public health.24,31 While genetic counseling assistants (GCAs) or extenders have been integrated in some clinics as a way to provide more time for GCs to practice at the top of their scope, the boundaries of practice for GCAs are not well defined. GCs typically agreed that data entry, coordinating samples, and administrative tasks were appropriate tasks for GCAs, and while some felt that GCAs should be able to return negative test results to patients there was general agreement that it would be inappropriate for GCAs to return abnormal test results.68,69,70
Laboratory GCs have emerged as a subspecialty and the main roles include liaising with patients or ordering providers, administrative duties, interpreting test results, and reviewing laboratory reports.71,72,73 Several studies described the involvement of GCs in test triage as part of laboratory utilization management.74,75,76,77 Also in the laboratory, CLGs can be the responsible head of a laboratory performing human genetics tests in 73% of the countries that recognize this title, according to a survey of more than 50 European and non-European countries.48 Responsibilities of the CLG vary by region, and can include writing and signing laboratory reports, results interpretation, teaching, and (less commonly) counseling patients.48
The studies above discuss the current scopes of practice for genetics providers; however, new testing technologies and applications, like GWS and direct-to-consumer testing, have the potential to impact the scopes of practice for these individuals. In a survey of Australian genetics specialists, GCs reported more pretest responsibilities and CGs were more involved in test interpretation tasks when GWS was used compared with when other tests were used.35 When surveyed about preferences for future roles, Australian GCs indicated a willingness to be involved in variant curation and classification, but follow-up interviews revealed this was with the goal of supporting patient care through better understanding of genomic test processes, rather than out of a desire to transition into laboratory GC roles.28 In another study, GCs were unsure if variant interpretation fell within their scope of practice but acknowledged that GWS would increase the complexity of their practices, although it would likely build on the core skill set that GCs already possess.58 Direct-to-consumer testing also impacted the scope of practice of genetics providers but there was uncertainty about what role GCs and CGs ought to play in counseling, interpreting, and confirming results or providing education for tests obtained in this way.78,79,80
Time spent on tasks
A time study of GCs in Michigan found that GCs spend 20% of their time on face-to-face interactions with patients, 64% on other patient-related activities (including case preparation, follow-up including documentation, and administrative tasks), and 16% on other tasks such as research and teaching.69 Notably, this study estimated that three hours of patient-related activities are performed for every 0.78 hours of face-to-face appointment time.69 Similarly, other studies have found that the most time consuming GC activities are patient-related activities (e.g., letter writing and documentation),69,81,82,83 though GCs in general practices spent more time on these than GCs in specialty practices.83
The time needed to provide pretest counseling varied widely depending on clinical setting, from less than ten minutes in prenatal screening to typically close to 1 hour when exome sequencing was performed (Supplementary Table B).35,81,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97 Typically, the median time for pretest counseling was 30–60 minutes.35,81 Factors that were associated with longer pretest counseling included joint appointments that included both a GC and a medical doctor, and pediatric testing.58,89,96 Shorter pretest counseling was associated with online, group, or telephone counseling; prenatal or cancer indications; and genetic counseling provided by nongenetics specialists.93,97 The time needed for post-test counseling appointments ranged from less than one minute for a negative prenatal screen93 to one to two hours when exome sequencing was performed.35,64,82,84,86,88,89,91,93,96,97,98 Workforce surveys reported that most post-test appointments are between 30 and 60 minutes35,64 and, in general, estimates of time spent on post-test counseling tended to be less than for pretest counseling, unless GWS was performed.35 Specifically, an Australian workforce survey reported that GWS took an additional 2.0 hours of GC time and 1.5 hours of CG time per patient compared with other tests, and CGs spent more time (~9.0 hours) than GCs (~8.5 hours) per GWS patient.28,35 Increased time spent on GWS patients is driven by the time needed to facilitate informed consent,58 convey complex results to patients,35 review medical records, prepare to discuss unfamiliar genetic results, and analyze and interpret test results.35,82,96,99
Case loads
The case loads reported in this section refer to the typical number of patients seen per month by each provider type. Genetic counselors had varied case loads that were highly influenced by specialty.35,69 The average monthly case loads for GCs seeing patients varied by country and study and were self-reported by GCs in Canada (averages of 26,32 30,36 and 44100), the United States (averages of 4069 and 51.9,95 with the latter including other modes of delivery than face to face), and Australia (2335). Of North American GCs who provided direct patient care, the highest monthly case loads were for those working in genomic profiling/personal genomics (i.e., use of genomic information without a clinical indication), preconception counseling, and assisted reproduction, whereas the lowest monthly case loads were in newborn screening and public health.64,95 The average monthly case loads for CGs also varied by country and study and were self-reported by CGs in the United States (averages of 7246 and 6881) and Australia (3135). A study from the United States compared data from 2015 to historical data and found that the patient case load for CGs had almost doubled, from 10 patients per week in 2005 to 18 patients per week in 2015.46
Scope of practice for nongenetics health-care providers
Nongenetics health-care providers (HCPs) are defined as providers who are not specifically trained as genetics providers but undertake genetics-related tasks. Ten studies assessed nongenetics HCP practices for family history taking and providing risk assessment for genetic disorders.63,87,101,102,103,104,105,106,107,108 Primary care providers, gastroenterologists and oncologists reported that they wanted standardized tools for taking family histories105 such as short family history questionnaires or electronic pedigree tools.102,106,107,108 However, when these tools were piloted, they did not appear to have a substantial impact on practice.106,107 A review article discussed genetics education interventions for primary care providers and found that education could lead to changes in knowledge and confidence but rarely translated to changes in practice.109 Five studies assessed nongenetics HCP preparedness for managing genetic information and found that the main concerns arose from uncertainty regarding clinical utility, lack of time, no existing workflows, and concerns about managing psychological impacts of genetic information.110,111,112,113,114
There were several studies about the practices of nongenetics HCPs in ordering genetic testing or referring their patients to a genetics specialist. Providers such as neurologists, psychiatrists, pulmonologists, dermatologists, and cardiologists were involved in ordering genetic testing, and the frequency and comfort level varied by setting.115,116,117,118,119,120 In studies that assessed referral patterns, between 9% and 58% of nongenetics HCPs reported that they had never referred a patient to a clinical genetics service for consultation.91,116,117,121 In these studies, neurologists and psychiatrists both had lower referral rates to genetics, but neurologists were more likely than psychiatrists to have ordered genetic testing.116,117,120 Overall, the main themes cited as barriers to referral were low perceived benefit for their patient, high costs, and limited availability of services.97,118,121,122,123 Two studies assessed the use of interventions to increase referral rates.124,125 One study demonstrated that the introduction of a Genetics Referral Toolkit designed specifically to target barriers to referral (which included a referral template, genetic risk checklist, and a family history worksheet) improved referral rates in a cancer setting.125 A second study introduced online educational modules to nongenetics HCPs, but although providers believed that they had increased their referral rates these remained unchanged after the intervention.124
The two main indications for which nongenetics HCPs provided genetic counseling were prenatal screening by obstetrician–gynecologists and hereditary cancer syndromes mainly by surgeons and oncologists.87,94,126,127,128,129,130,131,132,133,134 An assessment of the content of pretest counseling for prenatal screening by obstetrician–gynecologists found that they met the American College of Obstetricians and Gynecologists' recommendations for genetic counseling in only 1.1% of cases—notably, the disadvantages of screening were only discussed with 50% of patients.94,135 Several studies assessed the practices of nongenetics HCPs for genetic counseling for hereditary breast and ovarian cancer,87,126,131,132,133,134 with two studies finding that a significant portion of providers did not discuss the psychological impacts or the benefits and limitations of testing.87,126
One area of clinical care in which genetic testing by nongenetics HCPs has expanded is hereditary cancer, either by mainstreaming of a test (offering genetic testing in an oncology clinic, where pretest counseling and genetic test ordering would be done by an oncologist) or through rapid testing for individuals affected with cancer where results may impact treatment decisions. Most studies investigating attitudes found that the majority of providers believed that surgeons were the most appropriate providers of genetic testing.98,136,137 However, one survey of surgeons found that they did not believe it was their role to offer genetic testing and preferred to refer patients.138 While most studies found that oncology providers were positive about mainstreaming, others were concerned that mainstreaming would increase workload beyond capacity.138,139 Five studies that described oncologists’ ordering practices for genetic testing on tumor samples for treatment decision-making purposes found that oncologists tended to order more genetic tests140,141,142,143,144 than were recommended by professional guidelines.145
There were several additional studies that compared genetics providers and nongenetics HCPs.82,93,97,110,146,147,148,149,150,151,152 They described differences in provider knowledge,147 patient management,82,93,110,113,146,149,150,152 time and costs needed for tasks,82,93,97,150 and who was involved in providing care.60,82,93,97,146,151,152 Notably, one study identified and reported negative patient outcomes arising from nongenetics HCPs providing genetics services, such as psychological impacts on patients, insufficient counseling, inappropriate testing/screening, medical mismanagement, and poor health-care resource stewardship.110
Opportunities to increase capacity
Clinical genetics services have traditionally operated using a two-visit model with in-person pre- and post-test counseling appointments. Increased demand for services has led to the adoption of alternative service delivery models and technological innovations to enhance access and capacity. These include deviating from the traditional two-appointment counseling model (e.g., pretest only or post-test only),25,64,88,153 use of group genetic counseling,85,154 co-counseling by GCs and CGs,26,37,155 triage of patients for GC-only appointments,26,155 and using telehealth for counseling84,86,156,157,158,159,160,161 (Supplementary Table C). Genetics providers often operate using more than one service delivery model153,155 and adapt their approach in response to patient needs based on the complexity of the case26,37,155 and insurance requirements.155
Although alternative service delivery models allowed GCs to see more patients, some providers were concerned about a reduction in the quality of service.88 While group counseling was associated with shorter appointment times and was perceived as acceptable by patients, satisfaction was higher for individual counseling.85,154 Telehealth genetic counseling was found to increase access, reduce the cost of service, reduce wait times (at least in some studies), and be acceptable for patients (though less so for providers).84,86,156,157,158,159 One study showed that offering genetic counseling through a virtual clinic allowed 2.7 telehealth genetic counselors to cover the same patient load as 4 in-person counselors.159
As described previously, the two alternative service delivery models most used in specialty clinics were the mainstreaming of genetic testing and embedding a GC into interdisciplinary settings. Mainstreaming is common in oncology settings and has been shown to reduce wait times and decrease costs,98,137,138,139 while embedding a GC in an oncology or cardiology clinic increased the number of patients seen and decreased wait times and appointment length.92,162,163,164 Having a GC embedded in a cardiology clinic led to better identification and triage of patients for genetic counseling and also led to an increased referral rate for patients with syndromic features for a complete genetics consultation.163
Additional studies reported on quality improvement, task-sharing between different provider types, or information technology innovations such as automation of processes and online administrative tools. Some studies streamlined workflow processes or implemented a technical or automated element and then measured impact on capacity by assessing the time saved or impact on patient throughput.165,166,167,168,169,170,171 For example, one group developed a workflow for insurance preauthorizations, streamlining the process and reducing administrative tasks done by clinicians by delegating these tasks to nonclinicians.170 Overall, these interventions saved or redistributed time and were seen as satisfactory. Effective task-sharing through delegation of administrative or patient-related tasks to genetic counseling assistants or extenders was also reported as a way to enhance workflow by enabling GCs and CGs to focus on clinical tasks70,172 and see a higher volume of patients.68 Quality improvement through utilization management was also reported as a way to increase appropriate use of genetic services. GCs have been shown to play an important role in utilization management through patient identification and triage156,173 and through reviewing genetic test requests in a laboratory setting,74,75,76,77 resulting in a reduction in inappropriate testing.75,77
DISCUSSION
This review describes the composition of the clinical genetics workforce in high-income countries and identifies a range of factors that influence its capacity, including the number and types of relevant professionals, the scopes of practice of genetics and nongenetics specialists, patient case loads, time spent performing genetics tasks, and potential opportunities to increase efficiency. These factors are likely to be key drivers of the genetics workforce’s ability to meet the growing demand for clinical genetics services in the coming years. By summarizing relevant evidence, this review aims to inform and facilitate strategic planning by health-care systems to prepare for the expected future growth in the demand for genetics services.
A consistent theme in the literature is that the current capacity of the clinical genetics workforce is insufficient to meet existing demand for genetics services. Many of the reviewed studies pointed to an undersupply of genetics specialists, which can result in long wait times for routine referrals to CGs and GCs, ranging from a few months to over one year,46,139,156 and sometimes lead nongenetics professionals to be less likely to refer their patients to genetics clinics. However, these claims were not made in reference to a comprehensive evidence-based assessment of workforce needs, and there were limited data available on the CG and bioinformatics workforces in most high-income countries.21 Moreover, the types of outcomes reported were not standardized and tended to differ between the types of professions. The data on the CG workforce was limited to higher level surrogate outcomes compared with the more detailed metrics describing the GC workforce, and studies on nongenetics HCPs focused primarily on the education and skills required to deliver services rather than on metrics like case loads, wait times, and task completion time.
Policies aimed at increasing the size of the genetics workforce are on their own unlikely to succeed in boosting system capacity enough to meet current, let alone future, demand. For example, while the genetic counseling workforce has grown substantially in the last ten years, the number of nonclinical roles has also grown, so this has not directly translated into the same levels of growth in system capacity for direct patient care (e.g., only 59% of GCs in the United States working in direct patient care settings in 2019 compared with 84% in 2012).24,31 As a result, many of the publications in this review focus on innovative ways of working as a way of improving efficiency, which can expand capacity while maintaining the size of the workforce constant.
One approach to increasing the efficiency of the clinical genetics workforce is to implement policies to facilitate the ability of professionals to practice at “top of license” (e.g., the use of GCAs to ease the administrative burden on GCs). However, for this to be an effective strategy, broad agreement on the scope of practice for relevant professionals is necessary. While our literature review revealed general agreement that much of the accepted current scope of practice for GCs and CGs overlaps (with the exception of medical tasks such as physical examinations of patients and making diagnoses, which are acts reserved for CGs), there was uncertainty about how scope of practice would be impacted by broader clinical implementation of GWS. In addition, the models used for legal recognition of GCs in different jurisdictions can have a significant influence on the types of tasks that can be delegated or performed independently by GCs. Several different models of legal recognition and regulation of GCs are described in the literature,,3,27,35,36,37,39,55 and, although such recognition and regulation may enhance patient safety, the impact of different models on workforce capacity is unclear.
A second critical determinant of a health-care system’s overall capacity to provide genetics services is the role of nongenetics HCPs. Their involvement in taking family histories and conducting risk assessments, genetic counseling, and testing can increase capacity, but the evidence suggests that this task-sharing may be challenging to implement due to inconsistencies in willingness and competence to perform these tasks.174 This is illustrated by studies that evaluated the impacts of educational and technological interventions for primary care physicians and oncologists on increasing the identification and referral of genetically at-risk patients, which were usually found to have limited impact on practice behaviors. Additionally, the literature suggests that there are possible harms that can arise from nongenetics providers performing genetic counseling and testing.110,175,176,177 It is therefore imperative that nongenetics HCPs who do take more prominent roles in the provision of genetic counseling and testing are well prepared to provide these services to ensure appropriate patient ascertainment, testing, and follow-up care.
Finally, our review identified a range of initiatives undertaken to increase capacity through the use of more efficient service delivery models (e.g., incorporating decision aids) and the augmentation of services. This approach is likely to become increasingly important in the future as the development and use of electronic decision aids and artificial intelligence (e.g., chatbots) in clinical genetics services moves forward.178,179,180 Many studies highlighted the potential of alternative service delivery models, but while a recent systematic review of randomized controlled trials of outcomes of genetic counseling found that these can be as effective as in-person counseling in some settings (e.g., women at risk for hereditary cancer),181 it is important to emphasize that there remains a subset of patients for whom appropriate genetic counseling and testing will require the traditional in-person two-appointment model. Care needs to be taken when implementing efficiency improvement initiatives to ensure that appropriate services are available for all patients.
Ultimately, the rapidly changing landscape of genetics service provision, driven in part by the growing use of more complex tests like GWS, is likely to place additional strain on the capacity of the clinical genetics workforce. This review outlines what is currently known about its composition and capacity in high-income countries and aims to provide an evidence base for effective strategic workforce planning and policy development to address this challenge.
Change history
16 July 2020
The original version of this Article did not accurately document the participation of members of the GenCOUNSEL Study consortia in the list of authors. This has now been corrected in both the PDF and HTML versions of the Article.
17 July 2020
This Article was originally published without the accompanying supplementary files: Table A; Table B; and Table C. These file are now available in the HTML version of the Article; the PDF was correct from the time of publication.
11 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41436-020-0903-5
References
Phillips KA, Deverka PA, Hooker GW, Douglas MP. Genetic test availability and spending: where are we now? Where are we going? Health Aff. 2018;37:710–716.
De Sa J, et al. Growth of molecular diagnostics and genetic testing in the USA, 2008-2011: analysis and implications. Per Med. 2013;10:785–792.
DaVanzo J, Heath S, Pick A, Dobson A. Improving Medicare beneficiaries’ access to genetic counseling. 2013. https://www.abgc.net/abgc/media/documents/dobson-davanzo-report-to-nsgc_final-report-9-6-16.pdf. Accessed 21 March 2019.
Wetterstrand K. DNA sequencing costs: data from the NHGRI Genome Sequencing Program (GSP). 2018. http://www.genome.gov/sequencingcostsdata. Accessed 21 March 2019.
Retterer K, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016;18:696–704.
Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7:439–443.
Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: keeping up in the era of precision medicine. Am J Med Genet C. 2018;178:24–37.
Hoskovec JM, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27:16–20.
Elliott AM, Friedman JM. The importance of genetic counselling in genome-wide sequencing. Nat Rev Genet. 2018;19:735–736.
Bupp CP, Demmer LA, Saul RA. Surveying the current landscape of clinical genetics residency training. Genet Med. 2015;17:386–390.
Abacan MA, et al. The global state of the genetic counseling profession. Eur J Hum Genet. 2019;27:183–197.
Biesecker BB. Genetic counselors as social and behavioral scientists in the era of precision medicine. Am J Med Genet C. 2018;178:10–14.
Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–1334.
Unim B, et al. Identification of delivery models for the provision of predictive genetic testing in Europe: protocol for a multicentre qualitative study and a systematic review of the literature. Front Public Health. 2017;5:223.
Ingvoldstad C, et al. Components of genetic counsellor education: a systematic review of the peer-reviewed literature. J Community Genet. 2016;7:107–118.
Skirton H, Cordier C, Ingvoldstad C, Taris N, Benjamin C. The role of the genetic counsellor: a systematic review of research evidence. Eur J Hum Genet. 2015;23:452–458.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8:19–32.
Peters MDJ, Godfrey C, McInerney P, Baldini Soares C, Khalil H, Parker D. Chapter 11: Scoping reviews. In: Aromataris E, Munn Z (editors). Joanna Briggs Institute reviewer’s manual, JBI; 2017.
Tricco AC, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473.
Canadian Agency for Drugs and Technologies in Health. Grey matters: a practical tool for searching health-related grey literature. http://cadth.ca/resources/finding-evidence/grey-matters. Accessed 2 August 2018.
The World Bank. World Bank open data. https://data.worldbank.org/. Accessed 2 August 2018.
Margarit SB, Alvarado M, Alvarez K, Lay-son G. Medical genetics and genetic counseling in Chile. J Genet Couns. 2013;22:869–874.
Queensland Health. Statewide genetic health Queensland service plan 2017-2022. 2017. https://metronorth.health.qld.gov.au/rbwh/wp-content/uploads/sites/2/2019/08/ghq-service-plan-2017-22.pdf. Accessed 21 March 2019.
Dobson A, El-Gamil A, Pal S, Heath S, Davanzo J. Projecting the Supply and Demand for Certified Genetic Counselors: a Workforce Study. NSGC/ABGC Workforce Study. 2016.
Christensen J. Utah’s genetic counselor workforce, 2018: a study on the supply and distribution of genetic counselors in Utah. Utah Medical Education Council. 2018. https://umec.utah.gov/wp-content/uploads/Genetic-Counselor-Workforce-Report-2018.pdf. Accessed 21 March 2019.
Benjamin C, et al. A prospective cohort study assessing clinical referral management & workforce allocation within a UK regional medical genetics service. Eur J Hum Genet. 2015;23:996–1003.
Cordier C, Lambert D, Voelckel MAA, Hosterey-Ugander U, Skirton H. A profile of the genetic counsellor and genetic nurse profession in European countries. J Community Genet. 2012;3:19–24.
Nisselle A, et al. Readiness of clinical genetic healthcare professionals to provide genomic medicine: an Australian census. J Genet Couns. 2019;28:367–377.
Villegas C, Haga SB. Access to genetic counselors in the Southern United States. J Pers Med. 2019;9:E33. pii
National Society of Genetic Counselors. Professional status survey 2018: demographics and methodology. https://www.nsgc.org/p/cm/ld/fid=68. Accessed 10 April 2020.
National Society of Genetic Counselors. Professional status survey 2019: demographics and methodology. https://www.nsgc.org/p/cm/ld/fid=68. Accessed 10 April 2020.
Canadian Association of Genetic Counsellors. 2016 Professional status survey summary. https://www.cagc-accg.ca/doc/CAGC%202016%20PSS%20Summary.pdf. Accessed 10 April 2020.
Pan V, Yashar BM, Pothast R, Wicklund C. Expanding the genetic counseling workforce: program directors’ views on increasing the size of genetic counseling graduate programs. Genet Med. 2016;18:842–849.
Accreditation Council for Genetic Counseling. Annual accreditation report. 2018. https://www.gceducation.org/wp-content/uploads/2018/12/ACGC-17-18-AccredReport_FINAL.pdf. Accessed 21 March 2019.
Nisselle A, Macciocca I, McKenzie F. Professional status survey of genetic counsellors and clinical geneticists. Australian Genomics Health Alliance. 2018. https://www.australiangenomics.org.au/reports/professional-status-survey-of-genetic-counsellors-and-clinical-geneticists/. Accessed 21 March 2019.
Shugar AL, Quercia N, Trevors C, Rabideau MM, Ahmed S. Risk for patient harm in Canadian genetic counseling practice: it’s time to consider regulation. J Genet Couns. 2017;26:93–104.
Skirton H, et al. A study of the practice of individual genetic counsellors and genetic nurses in Europe. J Community Genet. 2013;4:69–75.
Paneque M. et al.The perceived impact of the European registration system for genetic counsellors and nurses. Eur J Hum Genet. 2017;25:1075–1077.
Barlow-Stewart K, Dunlop K, Fleischer R, Shalhoub C, Williams R. The NSW genetic counselling workforce. Sax Insitute for the NSW Institute of Health. 2015. https://www.saxinstitute.org.au/wp-content/uploads/The-NSW-Genetic-Counselling-Workforce_June2016.pdf. Accessed 21 March 2019.
Mendes Á, Sousa L, Paneque M. From constraints to opportunities? Provision of psychosocial support in Portuguese oncogenetic counseling services. J Genet Couns. 2013;22:771–783.
Cichon M, Feldman GL. Opportunities to improve recruitment into medical genetics residency programs: survey results of program directors and medical genetics residents. Genet Med. 2014;16:413–418.
British Columbia Medical Association. Doctors today and tomorrow. Planning British Columbia’s physician workforce. 2011. https://www.doctorsofbc.ca/sites/default/files/physicianworkforce_paper_web.pdf Accessed 10 April 2020.
Robboy S, et al. Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Arch Pathol Lab Med. 2013;137:1723–1732.
Federation of the Royal College of Physicians. Focus on physicians. 2018. https://www.rcplondon.ac.uk/projects/census-consultant-physicians-and-higher-specialty-trainees-uk. Accessed 21 March 2019.
Federation of the Royal College of Physicians. Focus on physicians. 2017. https://www.rcplondon.ac.uk/projects/census-consultant-physicians-and-higher-specialty-trainees-uk. Accessed 21 March 2019.
Maiese DR, Keehn A, Lyon M, Flannery D. Current conditions in medical genetics practice. Genet Med. 2018;21:1874–1877.
Liehr T, et al. European registration process for clinical laboratory geneticists in genetic healthcare. Eur J Hum Genet. 2017;25:515–519.
Liehr T, et al. Regarding the rights and duties of clinical laboratory geneticists in genetic healthcare systems; results of a survey in over 50 countries. Eur J Hum Genet. 2019;27:1168–1174.
Canadian Society for Medical Laboratory Science. Newly certified graduate employment survey. 2019. https://csmls.org/csmls/media/documents/resources/CSMLS-New-Graduate-Employment-Survey-2018-Grads-2017-v1-1.pdf. Accessed 21 March 2019.
Garcia E, Fisher PB. American Society for Clinical Pathology’s 2013 wage survey of medical laboratories in the United States. Lab Med. 2013;22:e97–e115.
Garcia E, Fisher PB. The American Society for Clinical Pathology’s 2015 wage survey of medical laboratories in the United States. Am J Clin Pathol. 2017;147:334–356.
Garcia E, Kundu I, Fong K. The American Society for Clinical Pathology’s 2017 wage survey of medical laboratories in the United States. Am J Clin Pathol. 2019;151:29–52.
Association for Clinical Genetic Science. Workforce Development Committee: Genetic Workforce Fig. 1–10. 2015. London, England. https://www.acgs.uk.com/workforce-development/. Accessed 21 March 2019.
Association for Clinical Genetic Science. Workforce Development Committee: Genetic Workforce Fig. 1–13. 2016. https://www.acgs.uk.com/workforce-development/. Accessed 21 March 2019.
Paneque M, Serra-Juhé C, Pestoff R, Cordier C, Silva J. Complementarity between medical geneticists and genetic counsellors: its added value in genetic services in Europe. Eur J Hum Genet. 2017;25:918–923.
Pestoff R, Ingvoldstad C, Skirton H. Genetic counsellors in Sweden: their role and added value in the clinical setting. Eur J Hum Genet. 2016;24:350–355.
Pestoff R, et al. How practical experiences, educational routes and multidisciplinary teams influence genetic counselors’ clinical practice in Europe. Clin Genet. 2018;93:891–898.
Dwarte T, Barlow K, Rosie S, Marcel OS, Terrill B. Role and practice evolution for genetic counseling in the genomic era: the experience of Australian and UK genetics practitioners. J Genet Couns. 2018;28:378–387.
Cordier C, Taris N, Moldovan R, Sobol H, Voelckel MAA. Genetic professionals’ views on genetic counsellors: a French survey. J Community Genet. 2016;7:51–55.
Gschmeidler B, Flatscher-Thoeni M. Ethical and professional challenges of genetic counseling—the case of Austria. J Genet Couns. 2013;22:741–752.
Barr JA, et al. Current practice for genetic counselling by nurses: an integrative review. Int J Nurs Pract. 2018;24:1–9.
Genetics in Nursing & Midwifery Task and Finish Group. Genetics/genomics in nursing and midwifery. UK National Health Service. 2011. https://www.gov.uk/government/publications/genetics-genomics-in-nursing-and-midwifery. Accessed 10 April 2020.
Calzone K, et al. National nursing workforce survey of nursing attitudes, knowledge and practice in genomics. Per Med. 2013;10:1–7.
National Society of Genetic Counselors. Professional status survey 2018: work environment. https://www.nsgc.org/p/cm/ld/fid=68.
Collis S, Gaff C, Wake S, McEwen A, McEwan A. Genetic counsellors and private practice: professional turbulence and common values. J Genet Couns. 2018;27:782–791.
Callard A, Newman W, Payne K. Delivering a pharmacogenetic service: is there a role for genetic counselors? J Genet Couns. 2012;21:527–535.
McWalter KM, Sdano MR, Dave G, Powell KP, Callanan N. Public health genetic counselors: activities, skills, and sources of learning. J Genet Couns. 2015;24:438–451.
Pirzadeh-Miller S, Robinson LS, Read P, Ross TS. Genetic counseling assistants: an integral piece of the evolving genetic counseling service delivery model. J Genet Couns. 2017;26:716–727.
Attard CA, Carmany EP, Trepanier AM. Genetic counselor workflow study: the times are they a-changin’? J Genet Couns. 2019;28:130–140.
Hnatiuk MJ, Noss R, Mitchell AL, Matthews AL. The current state of genetic counseling assistants in the United States. J Genet Couns. 2019;28:962–973.
Zetzsche LH, Kotzer KE, Wain KE. Looking back and moving forward: an historical perspective from laboratory genetic counselors. J Genet Couns. 2014;23:363–370.
Christian S, Lilley M, Hume S, Scott P, Somerville M. Defining the role of laboratory genetic counselor. J Genet Couns. 2012;21:605–611.
Waltman L, et al. Further defining the role of the laboratory genetic counselor. J Genet Couns. 2016;25:786–798.
Suarez CJ, Yu L, Downs N, Costa HA, Stevenson DA. Promoting appropriate genetic testing: the impact of a combined test review and consultative service. Genet Med. 2017;19:1049–1054.
Miller CE, et al. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A. 2014;164A:1094–1101.
Dickerson JA, et al. Improving the value of costly genetic reference laboratory testing with active utilization management. Arch Pathol Lab Med. 2014;138:110–113.
Wakefield E, et al. Reduction of health care costs and improved appropriateness of incoming test orders: the impact of genetic counselor review in an academic genetic testing laboratory. J Genet Couns. 2018;27:1067–1073.
McGowan ML, Fishman JR, Settersten RA, Lambrix MA, Juengst ET. Gatekeepers or intermediaries? The role of clinicians in commercial genomic testing. PLoS One. 2014;9:e108484.
Goldsmith L, Jackson L, Connor AO, Skirton H. Direct-to-consumer genomic testing from the perspective of the health professional: a systematic review of the literature. J Community Genet. 2013;4:169–180.
Haga SB, et al. Genomic risk profiling: attitudes and use in personal and clinical care of primary care physicians who offer risk profiling. J Gen Intern Med. 2011;26:834–840.
Sukenik-Halevy R, Ludman MD, Ben-Shachar S, Raas-Rothschild A. The time-consuming demands of the practice of medical genetics in the era of advanced genomic testing. Genet Med. 2016;18:372–377.
Lynch FL, et al. Time costs for genetic counseling in preconception carrier screening with genome sequencing. J Genet Couns. 2018;27:823–833.
Heald B, et al. Assessment of clinical workload for general and specialty genetic counsellors at an academic medical center: a tool for evaluating genetic counselling practices. npj Genomic Med. 2016;1:1–8.
McCuaig JM, et al. Modified panel-based genetic counseling for ovarian cancer susceptibility: a randomized non-inferiority study. Gynecol Oncol. 2019;153:108–115.
Cloutier M, et al. Group genetic counseling: an alternate service delivery model in a high risk prenatal screening population. Prenat Diagn. 2017;37:1112–1119.
Otten E, Birnie E, Ranchor AV, van Langen IM. Online genetic counseling from the providers’ perspective: counselors’ evaluations and a time and cost analysis. Eur J Hum Genet.2016;24:1255–1261.
Vadaaparampil S, Scherr C, Cragun D, Malo T, Pal T. Pretest genetic counseling services for hereditary breast and ovarian cancer delivered by nongenetics professionals in the state of Florida. Clin Genet. 2015;87:473–477.
Trepanier AM, Allain DC. Models of service delivery for cancer genetic risk assessment and counseling. J Genet Couns. 2014;23:239–253.
Heald B, Gustafson S, Mester J, Arscott P. A time study of cancer genetic counselors using a genetic counselor-only patient care model versus a traditional combined genetic counselor plus medical geneticist care model. J Natl Compr Cancer Netw. 2013;11:1076–1081.
Cohen SA, McIlvried DE. Impact of computer-assisted data collection, evaluation and management on the cancer genetic counselor’s time providing patient care. Fam Cancer. 2011;10:381–389.
Haga SB, et al. Primary care providers’ use of pharmacist support for delivery of pharmacogenetic testing. Pharmacogenomics. 2017;18:359–367.
Kentwell M, et al. Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol. 2017;145:130–136.
Miyake H, et al. Nationwide survey for current clinical status of amniocentesis and maternal serum marker test in Japan. J Hum Genet. 2016;61:879–884.
Colicchia L, et al. Patient–health care provider conversations about prenatal genetic screening: recommendation or personal choice. Obstet Gynecol. 2017;127:1145–1152.
National Society of Genetic Counselors. Professional status survey 2018: service delivery and access to care. https://www.nsgc.org/p/cm/ld/fid=68.
Arora S, Haverfield E, Richard G, Haga SB, Mills R. Clinical and counseling experiences of early adopters of whole exome sequencing. J Genet Couns. 2016;25:337–343.
Nishiyama M, Sawai H, Kosugi S. The current state of genetic counseling before and after amniocentesis for fetal karyotyping in Japan: a survey of obstetric hospital clients of a prenatal testing laboratory. J Genet Couns. 2013;22:795–804.
Douma KFL, et al. Health professionals’ evaluation of delivering treatment-focused genetic testing to women newly diagnosed with breast cancer. Fam Cancer. 2015;14:265–272.
Williams JL, Faucett WA, Smith-Packard B, Wagner M, Williams MS. An assessment of time involved in pretest case review and counseling for a whole genome sequencing clinical research program. J Genet Couns. 2014;23:516–521.
National Society of Genetic Counselors and Canadian Association of Genetic Counsellors. Professional status survey 2018: Canada. https://www.nsgc.org/p/cm/ld/fid=68.
Ahmed S, Hayward J, Ahmed M. Primary care professionals’ perceptions of using a short family history questionnaire. Fam Pract. 2016;33:704–708.
Owens KM, Marvin ML, Gelehrter TD, Iv MTR, Uhlmann WR. Clinical use of the Surgeon General’s “My Family Health Portrait” (MFHP) tool: opinions of future health care providers. J Genet Couns. 2011;20:510–525.
Wood ME, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;32:824–829.
Nippert I, et al. Cancer risk communication, predictive testing and management in France, Germany, the Netherlands and the UK: general practitioners’ and breast surgeons’ current practice and preferred practice responsibilities. J Community Genet. 2014;5:69–79.
Sperber NR, et al. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6:1–11.
Bell RA, et al. Impact of a randomized controlled educational trial to improve physician practice behaviors around screening for inherited breast cancer. J Gen Intern Med. 2015;30:334–341.
Zazove P, Plegue MA, Uhlmann WR. Prompting primary care providers about increased patient risk as a result of family history: does it work? J Am Board Fam Med. 2015;28:334–342.
Roter DL, et al. Effects of online genetics education on physician assistant interviewing skills. J Am Acad Physician Assist. 2010;25:36–48.
Paneque M, et al. A systematic review of interventions to provide genetics education for primary care. BMC Fam Pract. 2016;17:89.
Bensend TA, Veach PMC, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by nongenetics professionals. J Genet Couns. 2014;23:48–63.
Pet DB, et al. Physicians’ perspectives on receiving unsolicited genomic results. Genet Med. 2019;21:311–318.
Christensen K, et al. Are physicians prepared for whole genome sequencing? A qualitative analysis. Clin Genet. 2017;89:228–234.
Vassy JL, et al. The impact of whole genome sequencing on the primary care and outcomes of healthy adult patients: a pilot randomized trial. Ann Intern Med. 2018;167:159–169.
Laforest F, Kirkegaard P, Mann B, Edwards A. Genetic cancer risk assessment in general practice: systematic review of tools available, clinician attitudes, and patient outcomes. Br J Gen Pract. 2019;69:e97–e105.
Harris BU, Miyake CY, Motonaga KS, Dubin AM. Diagnosis and management of pediatric Brugada syndrome: a survey of pediatric electrophysiologists. Pacing Clin Electrophysiol. 2014;37:638–642.
Domingues-Carral J, et al. Genetic testing among Spanish pediatric neurologists: knowledge, attitudes and practices. Eur J Med Genet. 2017;60:124–129.
Wolfe K, et al. Genetic testing in intellectual disability psychiatry: opinions and practices of UK child and intellectual disability psychiatrists. J Appl Res Intellect Disabil. 2018;31:273–284.
Jacher JE, Martin LJ, Chung WK, Loyd JE, Nichols WC. Pulmonary arterial hypertension: specialists’ knowledge, practices, and attitudes of genetic counseling and genetic testing in the USA. Pulm Circ. 2017;7:372–383.
Shagalov DR, Ferzli GM, Wildman T, Glick SA. Genetic testing in dermatology: a survey analyzing obstacles to appropriate care. Pediatr Dermatol. 2017;34:33–38.
Salm M, et al. Use of genetic tests among neurologists and psychiatrists: knowledge, attitudes, behaviors, and needs for training. J Genet Couns. 2014;23:156–163.
Morrow A, et al. Referral of patients for pre-implantation genetic diagnosis: a survey of obstetricians. Aust N Z J Obstet Gynaecol. 2016;56:585–590.
Leach E, et al. How do physicians decide to refer their patients for psychiatric genetic counseling? A qualitative study of physicians’ practice. J Genet Couns. 2017;25:1235–1242.
Tan YY, Fitzgerald LJ. Barriers and motivators for referral of patients with suspected Lynch syndrome to cancer genetic services: a qualitative study. J Pers Med. 2014;4:20–34.
Houwink EJF, Muijtjens AMM, van Teeffelen SR, et al. Effect of comprehensive oncogenetics training interventions for general practitioners, evaluated at multiple performance levels. PLoS One. 2015;10:1–13.
Swanson CL, et al. Increasing genetic counseling referral rates through bundled interventions after ovarian cancer diagnosis. Gynecol Oncol. 2018;149:121–126.
Douma KFL, Smets EMA, Allain DC. Non-genetic health professionals’ attitude towards, knowledge of and skills in discussing and ordering genetic testing for hereditary cancer. Fam Cancer. 2016;15:341–350.
Musci TJ, et al. Non-invasive prenatal testing with cell-free DNA: US physician attitudes toward implementation in clinical practice. Prenat Diagn. 2013;33:424–428.
Amara N, et al. The knowledge value-chain of genetic counseling for breast cancer: an empirical assessment of prediction and communication processes. Fam Cancer. 2016;15:1–17.
Farrell RM, Agatisa PK, Mercer MB, Mitchum AG, Coleridge MB. The use of noninvasive prenatal testing in obstetric care: educational resources, practice patterns, and barriers reported by a national sample of clinicians. Prenat Diagn. 2016;36:499–506.
Benn P, et al. Obstetricians and gynecologists’ practice and opinions of expanded carrier testing and noninvasive prenatal testing. Prenat Diagn. 2014;34:145–152.
Beitsch PD, Whitworth PW. Can breast surgeons provide breast cancer genetic testing? An American Society of Breast Surgeons survey. Ann Surg Oncol. 2014;21:4104–4108.
Sussner KM, Jandorf L, Valdimarsdottir HB, Prevention C, City NY. Educational needs about cancer family history and genetic counseling for cancer risk among frontline healthcare clinicians in New York City. Genet Med. 2015;13:785–793.
Choi MC, et al. Practice patterns of hereditary ovarian cancer management in Korea. Int J Gynecol Cancer. 2017;27:895–899.
Tanabe N, Shikama A, Bando H. A survey of the practice patterns of gynecologic oncologists dealing with hereditary cancer patients in Japan. Fam Cancer. 2014;13:489–498.
ACOG Committee on Practice Bulletins. ACOG practice bulletin no. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007;109:217–227.
Burcher S, et al. Oncology health professionals attitudes toward treatment-focused genetic testing for women newly diagnosed with breast cancer. Per Med. 2013;10:431–440.
Wevers MR, et al. Rapid genetic counseling and testing in newly diagnosed breast cancer: patients’ and health professionals’ attitudes, experiences, and evaluation of effects on treatment decision making. J Surg Oncol. 2017;116:1029–1039.
Hallowell N, Stirling SWD, Porteous OYM. Moving into the mainstream: healthcare professionals’ views of implementing treatment focussed genetic testing in breast cancer care. Fam Cancer. 2019;18:293–301.
George A, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:1–8.
Gingras I, et al. The current use and attitudes towards tumor genome sequencing in breast cancer. Sci Rep. 2016;6:1–8.
Bar J, et al. EGFR mutation testing practice in advanced non-small cell lung cancer. Lung. 2014;192:759–763.
Arney J, et al. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer. 2018;116:25–29.
Gray SW, et al. Medical oncologists’ experiences in using genomic testing for lung and colorectal cancer care. J Oncol Pract. 2017;13:185–196.
Kim S, et al. Physician attitudes about genetic testing for localized prostate cancer: a national survey of radiation oncologists and urologists. Urol Oncol. 2018;36:501.e15–501.e21.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer V5. 2017. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
Falcone D, et al. Prenatal health care providers’ Gaucher disease carrier screening practices. Genet Med. 2012;14:844–851.
Gietel-Habets JJG, et al. Professionals’ knowledge, attitude and referral behaviour of preimplantation genetic diagnosis for hereditary breast and ovarian cancer. Reprod Biomed Online. 2018;36:137–144.
Roston TM, et al. The accessibility and utilization of genetic testing for inherited heart rhythm disorders: a Canadian cross-sectional survey study. J Community Genet. 2018;9:257–262.
Jayawardena ADL, Shearer AE, Smith RJH. Sensorineural hearing loss: a changing paradigm for its evaluation. Otolaryngol Head Neck Surg. 2015;153:843–850.
Lux MPM, et al. Time and resources needed to document patients with breast cancer from primary diagnosis to follow-up—results of a single-center study. Geburtshilfe Frauenheilkd. 2014;74:743–751.
Tanaka K, et al. Follow-up nationwide survey on predictive genetic testing for late-onset hereditary neurological diseases in Japan. J Hum Genet. 2013;58:560–563.
Cragun D, et al. Differences in BRCA counseling and testing practices based on ordering provider type. Genet Med. 2015;17:51–57.
Cohen SA, et al. Identification of genetic counseling service delivery models in practice: a report from the NSGC service delivery model task force. J Genet Couns. 2013;22:411–421.
Benusiglio PR, et al. Hereditary breast and ovarian cancer: successful systematic implementation of a group approach to genetic counselling. Fam Cancer. 2017;16:51–56.
Knapke S, Haidle JL, Nagy R, Pirzadeh-Miller S. The current state of cancer genetic counseling access and availability. Genet Med. 2016;18:410–412.
Kubendran S, Sivamurthy S, Schaefer GB. A novel approach in pediatric telegenetic services: geneticist, pediatrician and genetic counselor team. Genet Med. 2017;19:1260–1267.
Cohen SA, Huziak RC, Gustafson S, Grubs RE. Analysis of advantages, limitations, and barriers of genetic counseling service delivery models. J Genet Couns. 2016;25:1010–1018.
Buchanan AH, et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: cost, patient satisfaction, and attendance. J Genet Couns. 2015;24:961–970.
Weissman SM, Zellmer K, Gill N, Wham D. Implementing a virtual health telemedicine program in a community setting. J Genet Couns. 2018;27:323–325.
Terry AB, et al. Clinical models of telehealth in genetics: a regional telegenetics landscape. J Genet Couns. 2019;28:673–691.
Zierhut HA, Macfarlane IM, Ahmed Z, Davies J. Genetic counselors’ experiences and interest in telegenetics and remote counseling. J Genet Couns. 2018;27:329–338.
Tan RYC, et al. Using quality improvement methods and time-driven activity-based costing to improve value-based cancer care delivery at a cancer genetics clinic. J Oncol Pract. 2016;12:e320–e331.
Helm BM, et al. The genetic counselor in the pediatric arrhythmia clinic: review and assessment of services. J Genet Couns. 2018;27:558–564.
Senter L, et al. Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care. Gynecol Oncol. 2017;147:110–114.
Deisseroth CA, et al. ClinPhen extracts and prioritizes patient phenotypes directly from medical records to expedite genetic disease diagnosis. Genet Med. 2018;7:1585–1593.
Klinkenberg-Ramirez S, et al. Evaluation: a qualitative pilot study of novel information technology infrastructure to communicate genetic variant updates. Appl Clin Inform. 2016;7:461–476.
Lennerz JK, et al. Health care infrastructure for financially sustainable clinical genomics. J Mol Diagn. 2016;18:697–706.
Clark MM, et al. Diagnosis of genetic diseases in seriously ill children by rapid whole-genome sequencing and automated phenotyping and interpretation. Sci Transl Med. 2019;11:eaat6177.
Stark Z, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20:1554–1563.
Uhlmann WR, Schwalm K, Raymond VM. Development of a streamlined work flow for handling patients’ genetic testing insurance authorizations. J Genet Couns. 2017;26:657–668.
Patel D, Blouch EL, Rodgers-Fouché LH, Emmet MM, Shannon KM. Finding a balance: reconciling the needs of the institution, patient, and genetic counselor for optimal resource utilization. J Genet Couns. 2018;27:1318–1327.
Cohen SA, Nixon DM. A collaborative approach to cancer risk assessment services using genetic counselor extenders in a multi-system community hospital. Breast Cancer Res. Treat. 2016;159:527–534.
Eichmeyer JN, Burnham C, Sproat P, Tivis R, Beck TM. The value of a genetic counselor: improving identification of cancer genetic counseling patients with chart review. J Genet Couns. 2014;23:323–329.
Crellin E, et al. Preparing medical specialists to practice genomic medicine: education an essential part of a broader strategy. Front. Genet. 2019;10:789.
Brierley KL, et al. Adverse events in cancer genetic testing: medical, ethical, legal, and financial implications. Cancer J. 2012;18:303–309.
Bonadies DC, et al. Adverse events in cancer genetic testing: the third case series. Cancer J. 2014;20:246–253.
Farmer MB, et al. Adverse events in genetic testing: the fourth case series. Cancer J. 2019;25:231–236.
Birch P, et al. DECIDE: a decision support tool to facilitate parents’ choices regarding genome-wide sequencing. J Genet Couns. 2016;25:1298–1308.
Forbes Insights. Meet your new genetic counselor. 2019. https://www.forbes.com/sites/insights-intelai/2019/02/11/meet-your-new-genetic-counselor/#4f58ad09667c. Accessed 19 August 2019.
Rashkin MD, et al. Genetic counseling, 2030: an on-demand service tailored to the needs of a price conscious, genetically literate, and busy world. J Genet Couns. 2019;28:456–465.
Athens BA, et al. A systematic review of randomized controlled trials to assess outcomes of genetic counseling. J Genet Couns. 2017;26:902–933.
Acknowledgements
GenCOUNSEL was funded through the Large Scale Applied Research Project (LSARP) Genome Canada competition with cofunding from the Canadian Institutes of Health Research (CIHR), Genome BC, Genome Quebec, the BC Provincial Health Services Authority, BC Children’s Hospital Foundation and BC Women’s Hospital Foundation. The GenCOUNSEL Study is led by Alison M. Elliott, Jehannine Austin, Bartha Knoppers, and Larry D. Lynd with Project Manager Alivia Dey, and includes the following coinvestigators: Shelin Adam, Nick Bansback, Patricia Birch, Lorne Clarke, Nick Dragojlovic, Jan Friedman, Debby Lambert, Daryl Pullman, Alice Virani, Wyeth Wasserman, and Ma’n Zawati. We thank the Australian Genomics Health Alliance Workforce & Education program who collaborated with the Human Genetics Society of Australasia, Australasian Association of Clinical Geneticists, and Australasian Society of Genetic Counsellors to collect the Australasian census data. We also thank the Canadian Association of Genetic Counsellors and the National Society of Genetic Counselors for allowing us to include their workforce surveys in our review.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dragojlovic, N., Borle, K., Kopac, N. et al. The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Genet Med 22, 1437–1449 (2020). https://doi.org/10.1038/s41436-020-0825-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0825-2
Key words
This article is cited by
-
A survey of genetic and palliative care health professionals’ views of integrating genetics into palliative care
European Journal of Human Genetics (2024)
-
What is the power of a genomic multidisciplinary team approach? A systematic review of implementation and sustainability
European Journal of Human Genetics (2024)
-
The urgency for a change in genetics healthcare provision: views from Portuguese medical geneticists
Journal of Community Genetics (2024)
-
Evaluating implementation of NCCN guideline-directed genetic screening recommendations for patients with pancreatic ductal adenocarcinoma
Cancer Causes & Control (2024)
-
Empowerment of genetic information by women at-risk of being carriers of Duchenne and Becker muscular dystrophies
Journal of Community Genetics (2024)