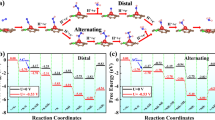
Abstract
The electrochemical ammonia synthesis has attracted increasing attention due to its energy saving characteristics. However, developing novel electrocatalysts and their mechanism remain great challenges. Here, several transition metal (TM) atoms doped on phosphorene were studied as N2 fixation electrocatalysts by using density functional theory (DFT) calculations. The results demonstrate that single Ru atom doped phosphorene shows an excellent catalytic activity for ammonia synthesis via the enzymatic pattern. A small overpotential of 0.696 V is achieved for this process. The effect of oxidation in the catalyst was also discussed in our work. Oxidation deactivates the catalyst, which should be avoided in the experiment. Our outcomes offer a novel perspective for single-atom catalytic ammonia synthesis with phosphorene as a substrate.
Graphic abstract







Similar content being viewed by others
References
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 2008;320(5878):889.
Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008;1(10):636.
Yang YL, Tang Y, Jiang HM, Chen YM, Wan PY, Fan MH, Zhang RR, Ullah S, Pan L, Zou JJ, Lao MM, Sun WP, Yang C, Zheng GF, Peng QL, Wang T, Luo YL, Sun XP, Konev AS, Levin OV, Lianos P, Hu ZF, Shen ZR, Zhao QL, Wang Y, Todorova N, Trapalis C, Sheridan MV, Wang HP, Zhang L, Sun SM, Wang WZ, Ma JM. 2020 roadmap on gas-involved photo- and electro-catalysis. Chin. Chem. Lett. 2019;30(12):2089.
Yang YL, Wu MG, Zhu XW, Xu H, Ma S, Zhi YF, Xia H, Liu XM, Pan J, Tang JY, Chai SP, Palmisano L, Parrino F, Liu JL, Ma JZ, Wang ZL, Tan L, Zhao YF, Song YF, Singh P, Raizada P, Jiang DL, Li D, Geioushy RA, Ma JZ, Zhang JT, Hu S, Feng RJ, Liu G, Liu MH, Li ZH, Shao MF, Li N, Peng JH, Ong WJ, Kornienko N, Xing ZY, Fan XJ, Ma JM. 2020 roadmap on two-dimensional nanomaterials for environmental catalysis. Chin. Chem. Lett. 2019;30(12):2065.
Wang QR, Guo JP, Chen P. Recent progress towards mild-condition ammonia synthesis. J. Energy Chem. 2019;36:25.
Giddey S, Badwal SPS, Kulkarni A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy. 2013;38(34):14576.
Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 2014;114(8):4041.
Cheng S, Gao YJ, Yan YL, Gao X, Zhang SH, Zhuang GL, Deng SW, Wei ZZ, Zhong X, Wang JG. Oxygen vacancy enhancing mechanism of nitrogen reduction reaction property in Ru/TiO2. J. Energy Chem. 2019;39:144.
Tanabe Y, Nishibayashi Y. Developing more sustainable processes for ammonia synthesis. Coord. Chem. Rev. 2013;257(17):2551.
Anderson JS, Rittle J, Peters JC. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature. 2013;501(7465):84.
MacLeod KC, Holland PL. Recent developments in the homogeneous reduction of dinitrogen by molybdenum and iron. Nat. Chem. 2013;5(7):559.
Schlögl R. Catalytic synthesis of ammonia—a “never-ending story”? Angew. Chem. Int. Ed. 2003;42(18):2004.
Daisley A, Hargreaves JSJ. The role of interstitial species upon the ammonia synthesis activity of ternary Fe–Mo–C(N) and Ni–Mo–C(N) phases. J. Energy Chem. 2019;39:170.
Abghoui Y, Garden AL, Hlynsson VF, Björgvinsdóttir S, Ólafsdóttir H, Skúlason E. Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design. Phys. Chem. Chem. Phys. 2015;17(7):4909.
Klerke A, Christensen CH, Nørskov JK, Vegge T. Ammonia for hydrogen storage: challenges and opportunities. J. Mater. Chem. 2008;18(20):2304.
Rittle J, Peters JC. An Fe–N2 complex that generates hydrazine and ammonia via Fe–NNH2: demonstrating a hybrid distal-to-alternating pathway for N2 reduction. J. Am. Chem. Soc. 2016;138(12):4243.
Rodriguez MM, Bill E, Brennessel WW, Holland PL. N2 reduction and hydrogenation to ammonia by a molecular iron-potassium complex. Science. 2011;334(6057):780.
Wei ZX, Feng YZ, Ma JM. Co-doped graphene edge for enhanced N2-to-NH3 conversion. J. Energy Chem. 2020;48:322.
Skúlason E, Bligaard T, Gudmundsdóttir S, Studt F, Rossmeisl J, Abild-Pedersen F, Vegge T, Jónsson H, Nørskov JK. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012;14(3):1235.
Yang YL, Liu JD, Wei ZX, Wang SY, Ma JM. Transition metal-dinitrogen complex embedded graphene for nitrogen reduction reaction. ChemCatChem. 2019;11(12):2821.
Ye KZZ, Shao J, Lin L, Gao D, Ta N, Si R, Wang G, Bao X. In situ reconstruction of a hierarchical Sn–Cu/SnOx core/shell catalyst for high-performance CO2 electroreduction. Angew. Chem. Int. Ed. 2020;132(12):4844.
Tanaka H, Nishibayashi Y, Yoshizawa K. Interplay between theory and experiment for ammonia synthesis catalyzed by transition metal complexes. Acc. Chem. Res. 2016;49(5):987.
Fu QR, Meng Y, Fang ZL, Hu QQ, Xu L, Gao WH, Huang XC, Xue Q, Sun YP, Lu FS. Boron nitride nanosheet-anchored Pd–Fe core–shell nanoparticles as highly efficient catalysts for suzuki–miyaura coupling reactions. ACS Appl. Mater. Interfaces. 2017;9(3):2469.
Hu SZ, Chen X, Li Q, Li FY, Fan ZP, Wang H, Wang YG, Zheng BH, Wu G. Fe3+ doping promoted N2 photofixation ability of honeycombed graphitic carbon nitride: the experimental and density functional theory simulation analysis. Appl. Catal. B: Environ. 2017;201:58.
Li XF, Li QK, Cheng J, Liu L, Yan Q, Wu Y, Zhang XH, Wang ZY, Qiu Q, Luo Y. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J. Am. Chem. Soc. 2016;138(28):8706.
Sun WL, Meng Y, Fu QR, Wang F, Wang GJ, Gao WH, Huang XC, Lu FS. High-yield production of boron nitride nanosheets and its uses as a catalyst support for hydrogenation of nitroaromatics. ACS Appl. Mater. Interfaces. 2016;8(15):9881.
Choi C, Back S, Kim NY, Lim J, Kim YH, Jung Y. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: a computational guideline. ACS Catal. 2018;8(8):7517.
Kattel S, Wang G. Reaction pathway for oxygen reduction on FeN4 embedded graphene. J. Phys. Chem. Lett. 2014;5(3):452.
Li QY, He LZ, Sun CH, Zhang XW. Computational study of MoN2 monolayer as electrochemical catalysts for nitrogen reduction. J. Phys. Chem. C. 2017;121(49):27563.
Mao X, Zhou S, Yan C, Zhu ZH, Du AJ. A single boron atom doped boron nitride edge as a metal-free catalyst for N2 fixation. Phys. Chem. Chem. Phys. 2019;21(3):1110.
Zhao JX, Chen ZF. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: a computational study. J. Am. Chem. Soc. 2017;139(36):12480.
Zhao WR, Zhang J, Zhu X, Zhang M, Tang J, Tan M, Wang Y. Enhanced nitrogen photofixation on Fe–doped TiO2 with highly exposed (101) facets in the presence of ethanol as scavenger. Appl. Catal. B: Environ. 2014;144:468.
Dinh KN, Zhang Y, Zhu J, Sun W. Phosphorene-based electrocatalysts. Chem. Eur J. 2020. https://doi.org/10.1002/chem.202000211.
Ou PF, Zhou X, Meng FC, Chen C, Chen YQ, Song J. Single molybdenum center supported on N-doped black phosphorus as an efficient electrocatalyst for nitrogen fixation. Nanoscale. 2019;11(28):13600.
Shi L, Li Q, Ling CY, Zhang YH, Ouyang YX, Bai XW, Wang JL. Metal-free electrocatalyst for reducing nitrogen to ammonia using a lewis acid pair. J. Mater. Chem. A. 2019;7(9):4865.
Zhang LL, Ding LX, Chen GF, Yang XF, Wang HH. Ammonia synthesis under ambient conditions: selective electroreduction of dinitrogen to ammonia on black phosphorus nanosheets. Angew. Chem. Int. Ed. 2019;58(9):2612.
Cheng YW, Song Y, Zhang YM. The doping and oxidation of 2D black and blue phosphorene: a new photocatalyst for nitrogen reduction driven by visible light. Phys. Chem. Chem. Phys. 2019;21(44):24449.
Liu K, Fu JW, Zhu L, Zhang XD, Li HM, Liu H, Hu JH, Liu M. Single-atom transition metals supported on black phosphorene for electrochemical nitrogen reduction. Nanoscale. 2020;12(8):4903.
Wei ZX, Zhang YF, Wang SY, Wang CY, Ma JM. Fe–doped phosphorene for the nitrogen reduction reaction. J. Mater. Chem. A. 2018;6(28):13790.
Tang X, Wei ZX, Liu QH, Ma JM. Strain engineering the d-band center for Janus MoSSe edge: nitrogen fixation. J. Energy Chem. 2019;33:155.
Kresse G, Furthmüller J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996;6(1):15.
Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B. 1993;47(1):558.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77(18):3865.
Liu CW, Li QY, Wu CZ, Zhang J, Jin YG, MacFarlane DR, Sun CH. Single-boron catalysts for nitrogen reduction reaction. J. Am. Chem. Soc. 2019;141(7):2884.
Montoya JH, Tsai C, Vojvodic A, Nørskov JK. The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. Chemsuschem. 2015;8(13):2180.
Zhu HR, Hu YL, Wei SH, Hua DY. Single-metal atom anchored on boron monolayer (β12) as an electrocatalyst for nitrogen reduction into ammonia at ambient conditions: a first-principles study. J. Phys. Chem. C. 2019;123(7):4274.
Zhao WH, Zhang LF, Luo QQ, Hu ZP, Zhang WH, Smith S, Yang JL. Single Mo1(Cr1) atom on nitrogen-doped graphene enables highly selective electroreduction of nitrogen into ammonia. ACS Catal. 2019;9(4):3419.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51302079, 51702138 and 51403193), the Natural Science Foundation of Hunan Province (No. 2017JJ1008) and the Key Research and Development Program of Hunan Province of China (No. 2018GK2031).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, JD., Wei, ZX., Dou, YH. et al. Ru-doped phosphorene for electrochemical ammonia synthesis. Rare Met. 39, 874–880 (2020). https://doi.org/10.1007/s12598-020-01451-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-020-01451-z