Abstract
Objective
To explore the value of olfactory identification deficits as a predictor of cerebral β-amyloid status and other markers of brain health in cognitively normal adults aged ~ 70 years.
Methods
Cross-sectional observational cohort study. 389 largely healthy and cognitively normal older adults were recruited from the MRC National Survey of Health and Development (1946 British Birth cohort) and investigated for olfactory identification deficits, as measured by the University of Pennsylvania Smell Identification Test. Outcome measures were imaging markers of brain health derived from 3 T MRI scanning (cortical thickness, entorhinal cortex thickness, white matter hyperintensity volumes); 18F florbetapir amyloid-PET scanning; and cognitive testing results. Participants were assessed at a single centre between March 2015 and January 2018.
Results
Mean (± SD) age was 70.6 (± 0.7) years, 50.8% were female. 64.5% had hyposmia and 2.6% anosmia. Olfaction showed no association with β-amyloid status, hippocampal volume, entorhinal cortex thickness, AD signature cortical thickness, white matter hyperintensity volume, or cognition.
Conclusion and relevance
In the early 70s, olfactory function is not a reliable predictor of a range of imaging and cognitive measures of preclinical AD. Olfactory identification deficits are not likely to be a useful means of identifying asymptomatic amyloidosis. Further studies are required to assess if change in olfaction may be a proximity marker for the development of cognitive impairment.
Similar content being viewed by others
Introduction
Simple, non-invasive markers of preclinical Alzheimer’s disease (AD) are needed. Odour identification (OI) deficits have been proposed as a potential risk marker for AD. Clinically, individuals diagnosed with AD and mild cognitive impairment (MCI) have poorer OI, and OI deficits are associated with cognitive decline and conversion to MCI and AD [1]; and AD pathology affects olfactory pathways in older adults [2] and animal models [3].
While the evidence for these associations in clinically defined groups is strong, the evidence regarding imaging biomarkers is more mixed. Table 1 summarises the previous literature investigating associations between OI and imaging markers of preclinical AD. Considering the two largest cohorts, Vassilaki et al. [4] and Growdon et al. [5] each found associations between poorer OI and imaging markers of neurodegeneration. Amyloid status was positively associated with poorer OI in the former, and at trend level in the latter study. In smaller studies, associations were not found [6, 7], or only seen when individuals with MCI or AD were included in pooled analyses [8, 9]. Associations between poorer OI and AD signature cortical thickness, and lower hippocampal volumes have been described [4, 7, 10, 11]. Associations with entorhinal cortex thickness or white matter hyperintensity volume have been present or absent in various studies [4,5,6, 10, 11].
A useful marker for preclinical AD would be positive early in the disease course, allowing a window for treatment. As the prevalence of AD pathology increases steeply with age, younger cohorts may be useful to investigate the earlier stages of disease.
In the current study, we explored associations between OI and markers of cerebral β-amyloid deposition (using 18F-florbetapir PET scanning), neurodegeneration, and cognition in a uniquely well-characterised cohort of near identical age drawn from the MRC National Survey of Health and Development (NSHD; the British 1946 birth cohort).
Methods
Participants
The Insight 46 study included 502 older adults recruited from the NSHD [12], a representative sample of singleton births in one week in March 1946 originally comprising 5326 individuals who have been followed prospectively throughout their lives [13]. Ethical approval was granted by the National Research Ethics Service Committee London (reference 14/LO/1173); participants provided written informed consent.
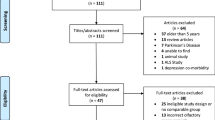
Participants attended a one-day visit at University College London between May 2016 and January 2018 (age 69–71 years). The cohort profile and recruitment information has been published [14]. We excluded participants without high-quality imaging (T1-weighted MRI and amyloid-PET), and those with mild cognitive impairment (MCI), neurodegenerative conditions, or conditions likely to affect olfactory function including previous sinus surgery or upper respiratory tract infection (Supplementary data).
Olfactory testing
The University of Pennsylvania Smell Identification Test (UPSIT) is a validated “scratch-and-sniff” test comprising 40 micro-encapsulated odorants, with four-option forced-choice answers [15]. Participants completed the “British” version at the study visit or soon thereafter. Where there was missing data for four or fewer items, a correction factor of 0.25 per missing item was applied, in line with other studies [16].
For categorical analyses, hyposmia was defined as UPSIT score ≤ 33 for males, ≤ 34 for females, and anosmia as UPSIT score ≤ 18 [15]. Normative data for the UPSIT British version have not been published; a comparison to norms for the UPSIT American version is shown in Table 1.
Neuropsychological testing
The cognitive battery included the Mini-Mental Status Examination (MMSE), Logical Memory, Digit-Symbol Substitution Test, and the Face-Name test [12]. These tests were combined into a modified version of the Preclinical Alzheimer Clinical Composite (PACC) score as described in Lu et al. [17].
Imaging
Participants underwent PET-MRI scanning on the same 3-T Siemens Biograph mMR scanner [12]. β-amyloid deposition was assessed over a 10-min period, 50 min after injection of 18F-florbetapir (370 mBq). A standardised uptake value ratio (SUVR) was generated from a grey matter cortical composite, with eroded white matter as the reference region. Gaussian mixture models determined a SUVR cut-point of 0.6104 to categorise binary amyloid status.
Hippocampal volume, entorhinal cortex thickness and AD signature cortical thickness were used as markers of neurodegeneration [4, 5]. Hippocampal volumes were determined using STEPS [18] with manual edits where appropriate. AD signature cortical thickness (a composite of temporal cortex regions as described in [19]) and entorhinal cortex measurements were determined using Freesurfer 6.0. Total intracranial volume was calculated using SPM12 (Statistical Parametric Mapping, https://www.fil.ion.ucl.ac.uk/spm/) [20]. White matter hyperintensity volume (WMHV) was derived using Bayesian Model Selection (BaMoS) [21].
Statistical methods
Data were analysed using Stata 14.1 (StataCorp LP). Chi-squared or Wilcoxon rank-sum tests were used for unadjusted analyses comparing OI category with binary or continuous demographic variables, respectively. Logistic regression was used for adjusted analysis of (binary) amyloid status, linear regression for hippocampal volumes, AD signature cortical thickness, entorhinal cortex thickness and PACC score. As WMHV was non-normally distributed, we used a general linear model with gamma log link. For each of these outcomes, we fitted models with continuous UPSIT score or OI impairment category as the predictor variable, and age, sex, and (where appropriate) TIV as covariates.
Results
Full data on 389 individuals were available for analysis: mean age at visit was 70.6 (SD 0.68) years, and 50.8% were female. Table 1 compares the distribution of UPSIT scores in this cohort with those of a large cohort of similar age assessed using the UPSIT (American version); the distribution of scores is similar.
Demographic and background features of the normosmic (32.9%), hyposmic (64.5%) and anosmic (2.6%) groups are shown in Table 1. There were no significant differences in sex, age, socio-economic position, smoking, history of head injury, ApoE4 status, or MMSE score between groups.
There was no significant relationship between continuous UPSIT score and binary amyloid status, adjusting for age and sex (OR 1.04, 95% CI 0.98–1.10, p = 0.24). There was no evidence that adding UPSIT score to a base model of age and sex improved prediction of amyloid status (Fig. 1).
Receiver operating characteristic curve illustrating the predictive value of age, sex and UPSIT score for amyloid status. Area under the curve for age and sex alone, 0.517 (95% confidence interval: 0.444–0.590), versus 0.545 (95% confidence interval 0.465–0.624) when UPSIT score is added to the model p = 0.466). This indicates that the addition of UPSIT score has very limited additional discriminatory value to predict amyloid status
Hippocampal volume, entorhinal cortex thickness, cortical thickness, PACC, or WMHV was not associated with continuous UPSIT scores, or when comparing groups categorically (Table 2) after adjusting for age and sex. There was similarly no relationship between UPSIT score and any of the components of the PACC (data not shown).
Discussion
In this study of 389 cognitively normal individuals around the age of 70 years, our main findings were (1) ~ 2/3 of individuals fulfil criteria for hyposmia, and (2) that there were no associations between low scores on olfactory identification testing and imaging evidence of β-amyloid pathology, neurodegeneration or cerebrovascular disease, or cognitive performance.
The strongest associations between olfaction and imaging metrics relevant to AD were reported in the Mayo Clinic cohort [4], which also has the highest average age (79 years). The Harvard cohort (mean age 74 years) [5] found a trend level association with amyloid status, and significant associations with imaging markers of neurodegeneration. Noting that our cohort was ~ 10 and 5 years younger than these, respectively, and as older individuals would be expected to have a shorter time to AD onset, this suggests that if OI impairment is not a useful screening tool for asymptomatic pathology, it may however be useful as a proximity marker for the emergence of cognitive impairment. The finding in smaller studies that associations between OI and imaging markers were strengthened by the inclusion of individuals with MCI (who are closer to disease onset) may also indicate this [8, 9]
Limitations of this study include its cross-sectional design and lack of a marker of tau pathology, as there is evidence from pathological [2] and biomarker [7, 22] studies that tau deposition may be more closely linked to olfactory changes. Longer term follow-up of this cohort and the addition of markers of tau pathology will be able to address the latter and the potential proximal relationship of OI to the development of cognitive impairment. Whether or not olfactory loss than can be seen in patients infected with Covid-19 relates to damage to olfactory epithelium or neuronal injury is the subject of ongoing debate, but at the current time, there is no evidence that this is related to Alzheimer pathology [23].
In summary, the high prevalence of OI impairment in populations at this age and lack of relationship between OI and markers of β-amyloid and neurodegeneration we find, indicate that the UPSIT is unlikely to be a reliable predictor of preclinical AD in its very earliest stages (Tables 3, 4).
Availability of data and material
A data-sharing policy is available on the NSHD Data Sharing website https://www.nshd.mrc.ac.uk/data.
References
Devanand DP, Lee S, Manly J et al (2015) Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84:182–189. https://doi.org/10.1212/wnl.0000000000001132
Wilson RS, Arnold SE, Schneider JA et al (2007) The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 78:30–35. https://doi.org/10.1136/jnnp.2006.099721
Cao L, Schrank BR, Rodriguez S et al (2012) Aβ alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat Commun 3:1009. https://doi.org/10.1038/ncomms2013
Vassilaki M, Christianson TJ, Mielke MM et al (2017) Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol 81:871–882. https://doi.org/10.1002/ana.24960
Growdon ME, Amariglio RE, Hedden T et al (2015) Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 84:2153–2160. https://doi.org/10.1212/WNL.0000000000001614
Dhilla Albers A, Asafu-Adjei J, Delaney MK et al (2016) Episodic memory of odors stratifies Alzheimer biomarkers in normal elderly. Ann Neurol 80:846–857. https://doi.org/10.1002/ana.24792
Risacher SL, Tallman EF, West JD et al (2017) Olfactory identification in subjective cognitive decline and mild cognitive impairment: association with tau but not amyloid positron emission tomography. Alzheimer’s Dement Diagn Assess Dis Monit 9:57–66. https://doi.org/10.1016/j.dadm.2017.09.001
Kreisl WC, Jin P, Lee S et al (2018) Odor identification ability predicts PET amyloid status and memory decline in older adults. J Alzheimer’s Dis 62:1759–1766. https://doi.org/10.3233/JAD-170960
Bahar-Fuchs A, Chételat G, Villemagne VL et al (2010) Olfactory deficits and amyloid-β burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: A PiB PET study. J Alzheimer’s Dis 22:1081–1087. https://doi.org/10.3233/JAD-2010-100696
Devanand DP, Tabert MH, Cuasay K et al (2010) Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging 31:1593–1600. https://doi.org/10.1016/j.neurobiolaging.2008.09.008
Heinrich J, Vidal JS, Simon A et al (2018) Relationships between lower olfaction and brain white matter lesions in elderly subjects with mild cognitive impairment. J Alzheimer’s Dis 61:1133–1141. https://doi.org/10.3233/JAD-170378
Lane CA, Parker TD, Cash DM et al (2017) Study protocol: Insight 46 - a neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol 17:75. https://doi.org/10.1186/s12883-017-0846-x
Wadsworth M, Kuh D, Richards M, Hardy R (2006) Cohort profile: the 1946 national birth cohort (MRC National Survey of Health and Development). Int J Epidemiol 35:49–54. https://doi.org/10.1093/ije/dyi201
James S-N, Lane CA, Parker TD et al (2018) Using a birth cohort to study brain health and preclinical dementia: recruitment and participation rates in Insight 46. BMC Res Notes 11:885. https://doi.org/10.1186/s13104-018-3995-0
Doty RL, Shaman P, Kimmelman CP, Dann MS (1984) University of pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 94:176–178. https://doi.org/10.1288/00005537-198402000-00004
Wilson RS, Schneider JA, Arnold SE et al (2007) Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry 64:802. https://doi.org/10.1001/archpsyc.64.7.802
Lu K, Nicholas JM, Collins JD et al (2019) Cognition at age 70: life course predictors and associations with brain pathologies. Neurology 93:e2144–e2156. https://doi.org/10.1212/WNL.0000000000008534
Jorge Cardoso M, Leung K, Modat M et al (2013) STEPS: similarity and truth estimation for propagated segmentations and its application to hippocampal segmentation and brain parcelation. Med Image Anal 17:671–684. https://doi.org/10.1016/j.media.2013.02.006
Jack CR, Wiste HJ, Weigand SD et al (2015) Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 138:3747–3759. https://doi.org/10.1093/brain/awv283
Malone IB, Leung KK, Clegg S et al (2015) Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 104:366–372. https://doi.org/10.1016/j.neuroimage.2014.09.034
Sudre CH, Cardoso MJ, Bouvy W et al (2015) Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging 34:2079–2102
Lafaille-Magnan M-E, Poirier J, Etienne CP et al (2017) Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89:327–335. https://doi.org/10.1212/WNL.0000000000004954
Zubair AS, McAlpine LS, Gardin T et al (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.2065
Doty RL (1995) The smell identification test (TM) administration manual. Sensonics Inc, Philadelphia
Funding
This study is principally funded by grants from Alzheimer’s Research UK (grants ARUK-PG2014-1946 and ARUK-PG2017-1946), the Medical Research Council Dementias Platform UK (Grant CSUB19166), and the Wolfson Foundation (Grant PR/ylr/18575). The genetic analyses are funded by the Brain Research Trust (Grant UCC14191). The florbetapir amyloid tracer was provided by Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly). The National Survey of Health and Development and Drs Richards and Wong are funded by the Medical Research Council (Grants MC_UU_12019/1 and MC_UU_12019/3). Additional support was received from the National Institutes of Health Research Queen Square Dementia BRU (Drs Schott and Fox), University College London Hospitals Biomedical Research Centre (Dr Schott), Leonard Wolfson Experimental Neurology Centre (Drs Schott, Fox and Thomas), a Wellcome Trust Clinical Research Fellowship (200109/Z/15/Z [Dr Parker]), Leonard Wolfson Experimental Neurology Clinical Research Fellowship (525367 [Dr Keshavan]), an Alzheimer’s Society Junior Fellowship (AS-JF-17-011 [Dr Sudre]), Medical Research Council (Dr Fox), the UK Dementia Research Institute at UCL (Dr Fox), an National Institutes of Health Researcher Senior Investigator award (Dr Fox), the NIA (Dr Fox), and the EPSRC (EP/J020990/1 [Dr Schott] and a separate award to Dr Fox), and European Union’s Horizon 2020 research and innovation program (grant 666992 [Dr Schott]).The funders and sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr Fox’s research group has received payment for consultancy or for conducting studies from Avid Radiopharmaceuticals, Biogen, Eli Lilly Research Laboratories, General Electric Healthcare, and Roche; Dr Fox receives no personal compensation for these activities. Dr Schott reports grants from Weston Brain Foundation during the conduct of the study and personal fees from Axon Neuroscience, Roche, Eli Lilly, General Electric Healthcare, Merck Sharp & Dohme, Oxford University Press, Biogen, and EU Horizon 2020 outside the submitted work.
Ethics approval
Ethical approval was granted by National research ethics Service Committee London (reference 14/LO/1173), and the study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Participants provided written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buchanan, S.M., Parker, T.D., Lane, C.A. et al. Olfactory testing does not predict β-amyloid, MRI measures of neurodegeneration or vascular pathology in the British 1946 birth cohort. J Neurol 267, 3329–3336 (2020). https://doi.org/10.1007/s00415-020-10004-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10004-4