Abstract
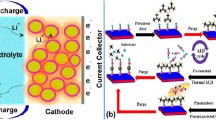
Microwave radiation (MWR), a type of electromagnetic excitation source, reduces the synthesis temperature and processing time for chemical reactions compared to traditional synthesis methods. Recently, we demonstrated that MWR can engineer ceramics with different crystal phases compared to traditional methods [Journal of Materials Chemistry A5, 35 (2017)]. In this study, we further apply the MWR-assisted technique to improve the electrochemical performance of LiCoO2 cathodes by engineering TiO2 and ZrO2 ceramic coatings. Electrochemical tests suggest that the TiO2 coating improves the rate capability of the LiCoO2 electrode. Both TiO2 and ZrO2 coatings improve the high-voltage (4.5 V) cycling stability of LiCoO2. The capacity remaining is improved from 52.8 to 84.4% and 81.9% by the TiO2 coating and the ZrO2 coating, respectively, after 40 cycles. We compare these results with existing studies that apply traditional methods to engineer TiO2/ZrO2 on LiCoO2, and find that the MWR-assisted method shows better performance improvement. X-ray photoelectron spectroscopy measurements suggest that the improved cycling stability arises from the formation of metal fluorides that protect the electrode from side reactions with electrolytes. This mechanism is further supported by the reduced Co dissolution from TiO2/ZrO2-coated LiCoO2 electrode after cycling. This study provides a new toolbox facilitating the integration of many delicate, low melting point materials like polymers into battery electrodes.









Similar content being viewed by others
References
Kalluri S, Yoon M, Jo M et al (2017) Surface engineering strategies of layered LiCoO2 cathode material to realize high-energy and high-voltage Li–ion cells. Adv energy mater 7:1601507
Su L, Smith PM, Anand P et al (2018) Surface engineering of a LiMn2O4 electrode using nanoscale polymer thin films via chemical vapor deposition polymerization. ACS Appl Mater Inter 10:27063–27073
Zhou A, Liu Q, Wang Y et al (2017) Al2O3 surface coating on LiCoO2 through a facile and scalable wet-chemical method towards high-energy cathode materials withstanding high cutoff voltages. J Mater Chem A 5:24361–24370
Dai X, Wang L, Xu J et al (2014) Improved electrochemical performance of LiCoO2 electrodes with ZnO coating by radio frequency magnetron sputtering. ACS Appl Mater InteR 6:15853–15859
Dai X, Zhou A, Xu J et al (2015) Superior electrochemical performance of LiCoO2 electrodes enabled by conductive Al2O3-doped ZnO coating via magnetron sputtering. J Power Sour 298:114–122
Zhou A, Lu Y, Wang Q et al (2017) Sputtering TiO2 on LiCoO2 composite electrodes as a simple and effective coating to enhance high-voltage cathode performance. J Power Sour 346:24–30
Zhou A, Xu J, Dai X et al (2016) Improved high-voltage and high-temperature electrochemical performances of LiCoO2 cathode by electrode sputter-coating with Li3PO4. J Power Sour 322:10–16
Wang L, Chen B, Ma J et al (2018) Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem Soc Rev 47:6505–6602
Jha, S. K.; Phuah, X. L.; Luo, J. et al. (2018), The effects of external fields in ceramic sintering., J AM CERAM SOC
Lidström P, Tierney J, Watheyb B et al (2001) Microwave assisted organic synthesis - a review. Tetrahedron 57:9225–9283
Nakamura, N.; Terban, M. W.; Billinge, S. et al. (2017), Unlocking the structure of mixed amorphous-crystalline ceramic oxide films synthesized under low temperature electromagnetic excitation., J Mater Chem A
Reeja-Jayan, B.; Harrison, K. L.; Yang, K. et al. (2012), Microwave-assisted Low-temperature Growth of Thin Films in Solution., Sci Rep-UK 2:
Bondioli F, Ferrari AM, Leonelli C et al (2001) Microwave-hydrothermal synthesis of nanocrystalline zirconia powders. J Am Ceram Soc 84:2728–2730
Shah JJ, Mohanraj K (2014) Comparison of conventional and microwave-assisted synthesis of benzotriazole derivatives. Indian J Pharm Sci 76:46
Kim KC, Jegal J, Bak S et al (2014) Improved high-voltage performance of FePO4-coated LiCoO2 by microwave-assisted hydrothermal method. Electrochem Commun 43:113–116
Hoogenboom R, Schubert US (2007) Microwave-assisted polymer synthesis: recent developments in a rapidly expanding field of research. Macromol Rapid Commun 28:368–386
Su L, Tong P, Zhang L et al (2019) Photoelectrochemical immunoassay of aflatoxin B1 in foodstuff based on amorphous TiO2 and CsPbBr 3 perovskite nanocrystals. Analyst 144:4880–4886
Shu J, Qiu Z, Lv S et al (2018) Plasmonic enhancement coupling with defect-engineered TiO2–x: a mode for sensitive photoelectrochemical biosensing. Anal Chem 90:2425–2429
Kim YJ, Cho J, Kim T et al (2003) Suppression of cobalt dissolution from the LiCoO2 cathodes with various metal-oxide coatings. J Electrochem Soc 150:1723
Nakamura N, Seepaul J, Kadane JB et al (2017) Design for low-temperature microwave-assisted crystallization of ceramic thin films. Appl Stoch Model Bus 33:314–321
Jha SK, Charalambous H, Okasinski JS et al (2019) Using in operando diffraction to relate lattice strain with degradation mechanism in a NMC battery. J Mater Sci 54:2358–2370.https://doi.org/10.1007/s10853-018-3007-8
Jha SK, Nakamura N, Zhang S et al. (2019) Defect‐mediated anisotropic lattice expansion in ceramics as evidence for nonthermal coupling between electromagnetic fields and matter. Adv Eng Mater 1900762
Nakamura NJ, Culbertson E, Billinge SJ et al. (2019) The role of defects in microwave-assisted synthesis of cubic ZrO2
Nam KM, Shim JH, Han D et al (2010) Syntheses and characterization of wurtzite CoO, rocksalt CoO, and spinel Co3O4 nanocrystals: their interconversion and tuning of phase and morphology. Chem Mater 22:4446–4454
Sun Y, Han J, Myung S et al (2006) Significant improvement of high voltage cycling behavior AlF3-coated LiCoO2 cathode. Electrochem Commun 8:821–826
Schulz N, Hausbrand R, Wittich C et al (2018) XPS-surface analysis of sei layers on Li–Ion cathodes: part II. SEI-composition and formation inside composite electrodes. J Electrochem Soc 165:A833–A846
Schulz N, Hausbrand R, Dimesso L et al (2018) XPS-surface analysis of sei layers on Li–Ion cathodes: Part I. Investigation of initial surface chemistry. J Electrochem Soc 165:A819–A832
Yang Z, Li R, Deng Z (2018) A deep study of the protection of lithium cobalt oxide with polymer surface modification at 4.5 V high voltage. Sci Rep-UK
Bai Y, Liu N, Liu J et al (2006) Coating Material-Induced Acidic Electrolyte Improves LiCoO2 Performances. Electrochem Solid-State Lett 9:A552
Aurbach D, Markovsky B, Rodkin A et al (2002) On the capacity fading of LiCoO2 intercalation electrodes: the effect of cycling, storage, temperature, and surface film forming additives. Electrochim Acta 47:4291–4306
Miyashiro H, Yamanaka A, Tabuchi M et al (2006) Improvement of degradation at elevated temperature and at high state-of-charge storage by ZrO2 coating on LiCoO2. J Electrochem Soc 153:A348–A353
Cheng H, Wang F, Chu JP et al (2012) Enhanced cycleabity in lithium ion batteries: resulting from atomic layer depostion of Al2O3 or TiO2 on LiCoO2 electrodes. J Phys Chem C 116:7629–7637
Jayasree SS, Nair S, Santhanagopalan D (2018) Ultrathin TiO2 coating on LiCoO2 for improved electrochemical performance as Li–ion battery cathode. CHEMISTRYSELECT 3:2763–2766
Hwang BJ, Chen CY, Cheng MY et al (2010) Mechanism study of enhanced electrochemical performance of ZrO2-coated LiCoO2 in high voltage region. J Power Sour 195:4255–4265
Chung KY, Yoon W, Lee HS et al (2006) In situ XRD studies of the structural changes of ZrO2-coated LiCoO2 during cycling and their effects on capacity retention in lithium batteries. J Power Sour 163:185–190
Yu J, Han Z, Hu X et al (2014) The investigation of Ti-modified LiCoO2 materials for lithium ion battery. J Power Sou 262:136–139
Zhang J, Wang R, Yang X et al (2012) Direct observation of inhomogeneous solid electrolyte interphase on MnO anode with atomic force microscopy and spectroscopy. Nano Lett 12:2153–2157
Kerlau M, Kostecki R (2006) Interfacial impedance study of Li–ion composite cathodes during aging at elevated temperatures. J Electrochem Soc 153:1644
Acknowledgements
This work was supported by National Science Foundation (NSF) CAREER Award (CMMI1751605) and INCUBATE seed funding from Carnegie Mellon University. Microwave field-assisted TiO2 synthesis efforts were supported by the DARPA AIRA Program (Grant Number HR00111990030). The authors acknowledge use of the Materials Characterization Facility at Carnegie Mellon University supported by Grant MCF-677,785. The Authors thank Dr. Joel Gillespie from the University of Pittsburgh Materials Characterization Laboratory for the access to the XPS spectrometer. Dr. Haiyan Wang and Ms. Xin Li Phuah acknowledge the support from the Office of Naval Research under Contract Nos. 1. N00014-17-1-2087 and N00014-16-1-2778. This research used 6-ID-D beamline and other resources in Advanced Photon Source, Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
LS contributed to conceptualization, experimental design, carrying out measurements, manuscript composition. SKJ helped in carrying out measurements. XLP involved in carrying out measurements. JX contributed to carrying out measurements. NN helped in experimental design, manuscript composition. HW helped in resources, funding acquisition. JSO contributed to resources. BRJ helped in supervision, experimental design, resources, manuscript composition, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, L., Jha, S.K., Phuah, X.L. et al. Engineering lithium-ion battery cathodes for high-voltage applications using electromagnetic excitation. J Mater Sci 55, 12177–12190 (2020). https://doi.org/10.1007/s10853-020-04871-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04871-5