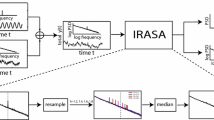
Abstract
Cortical neural networks maintain autonomous electrical activity called spontaneous activity that represents the brain’s dynamic internal state even in the absence of sensory stimuli. The spatio-temporal complexity of spontaneous activity is strongly related to perceptual, learning, and cognitive brain functions; multi-fractal analysis can be utilized to evaluate the complexity of spontaneous activity. Recent studies have shown that the deterministic dynamic behavior of spontaneous activity especially reflects the topological neural network characteristics and changes of neural network structures. However, it remains unclear whether multi-fractal analysis, recently widely utilized for neural activity, is effective for detecting the complexity of the deterministic dynamic process. To verify this point, we focused on the log-normal distribution of excitatory postsynaptic potentials (EPSPs) to evaluate the multi-fractality of spontaneous activity in a spiking neural network with a log-normal distribution of EPSPs. We found that the spiking activities exhibited multi-fractal characteristics. Moreover, to investigate the presence of a deterministic process in the spiking activity, we conducted a surrogate data analysis against the time-series of spiking activity. The results showed that the spontaneous spiking activity included the deterministic dynamic behavior. Overall, the combination of multi-fractal analysis and surrogate data analysis can detect deterministic complex neural activity. The multi-fractal analysis of neural activity used in this study could be widely utilized for brain modeling and evaluation methods for signals obtained by neuroimaging modalities.


Similar content being viewed by others
References
Adeli H, Ghosh-Dastidar S, Dadmehr N (2005a) Alzheimer’s disease and models of computation: imaging, classification, and neural models. J Alzheimers Dis 7(3):187–199
Adeli H, Ghosh-Dastidar S, Dadmehr N (2005b) Alzheimer’s disease: models of computation and analysis of EEGs. Clin EEG Neurosci 36(3):131–140
Adeli H, Ghosh-Dastidar S, Dadmehr N (2008) A spatio-temporal wavelet-chaos methodology for eeg-based diagnosis of Alzheimer’s disease. Neurosci Lett 444(2):190–194
Bellec G, Salaj D, Subramoney A, Legenstein R, Maass W (2018) Long short-term memory and learning-to-learn in networks of spiking neurons. In: Advances in neural information processing systems, pp 787–797
Bonzon P (2017) Towards neuro-inspired symbolic models of cognition: linking neural dynamics to behaviors through asynchronous communications. Cogn Neurodyn 11(4):327–353
Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG (2011) Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Nat Acad Sci 108(40):16783–16788
Buzsáki G, Mizuseki K (2014) The log-dynamic brain: how skewed distributions affect network operations. Nat Rev Neurosci 15(4):264–278
da Silva FL (2013) EEG and MEG: relevance to neuroscience. Neuron 80(5):1112–1128
Destexhe A (2009) Self-sustained asynchronous irregular states and up–down states in thalamic, cortical and thalamocortical networks of nonlinear integrate-and-fire neurons. J Comput Neurosci 27(3):493
Easwaramoorthy D, Uthayakumar R (2010) Analysis of biomedical EEG signals using wavelet transforms and multifractal analysis. In: 2010 international conference on communication control and computing technologies, IEEE, pp 544–549
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700
Garrett DD, Kovacevic N, McIntosh AR, Grady CL (2011) The importance of being variable. J Neurosci 31(12):4496–4503
Gautama T, Mandic DP, Van Hulle MM (2003) Indications of nonlinear structures in brain electrical activity. Phys Rev E 67(4):046204
Goodman DF, Stimberg M, Yger P, Brette R (2014) Brian 2: neural simulations on a variety of computational hardware. BMC Neurosci 15(1):P199
Guo D, Li C (2010) Self-sustained irregular activity in 2-D small-world networks of excitatory and inhibitory neurons. IEEE Trans Neural Netw 21(6):895–905
Hahn G, Ponce-Alvarez A, Monier C, Benvenuti G, Kumar A, Chavane F, Deco G, Frégnac Y (2017) Spontaneous cortical activity is transiently poised close to criticality. PLoS Comput Biol 13(5):e1005543
Hasegawa C, Takahashi T, Yoshimura Y, Ikeda T, Saito DN, Kumazaki H, Minabe Y, Kikuchi M (2018) Developmental trajectory of infant brain signal variability: a longitudinal pilot study. Front Neurosci 12:566
Jaffard S, Lashermes B, Abry P (2006) Wavelet leaders in multifractal analysis. In: Wavelet analysis and applications, Springer, pp 201–246
Kanamaru T (2017) Chaotic pattern alternations can reproduce properties of dominance durations in multistable perception. Neural Comput 29(6):1696–1720
Kantz H, Schreiber T (2004) Nonlinear time series analysis, vol 7. Cambridge University Press, Cambridge
Kheradpisheh SR, Ganjtabesh M, Thorpe SJ, Masquelier T (2018) STDP-based spiking deep convolutional neural networks for object recognition. Neural Netw 99:56–67
Kim SY, Lim W (2017) Dynamical responses to external stimuli for both cases of excitatory and inhibitory synchronization in a complex neuronal network. Cogn Neurodyn 11(5):395–413
Kim SY, Lim W (2018) Effect of spike-timing-dependent plasticity on stochastic burst synchronization in a scale-free neuronal network. Cogn Neurodyn 12(3):315–342
Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R (2007) Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev 31(7):1003–1016
Kulkarni SR, Rajendran B (2018) Spiking neural networks for handwritten digit recognition—supervised learning and network optimization. Neural Netw 103:118–127
La Rocca D, Zilber N, Abry P, van Wassenhove V, Ciuciu P (2018) Self-similarity and multifractality in human brain activity: a wavelet-based analysis of scale-free brain dynamics. J Neurosci Methods 309:175–187
Lee JH, Delbruck T, Pfeiffer M (2016) Training deep spiking neural networks using backpropagation. Front Neurosci 10:508
Lin X, Wang X, Hao Z (2017) Supervised learning in multilayer spiking neural networks with inner products of spike trains. Neurocomputing 237:59–70
Lin Z, Ma D, Meng J, Chen L (2018) Relative ordering learning in spiking neural network for pattern recognition. Neurocomputing 275:94–106
Maksimenko VA, Pavlov A, Runnova AE, Nedaivozov V, Grubov V, Koronovslii A, Pchelintseva SV, Pitsik E, Pisarchik AN, Hramov AE (2018) Nonlinear analysis of brain activity, associated with motor action and motor imaginary in untrained subjects. Nonlinear Dyn 91(4):2803–2817
McCormick DA (1999) Spontaneous activity: signal or noise? Science 285(5427):541–543
Mizuno T, Takahashi T, Cho RY, Kikuchi M, Murata T, Takahashi K, Wada Y (2010) Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin Neurophysiol 121(9):1438–1446
Mozafari M, Kheradpisheh SR, Masquelier T, Nowzari-Dalini A, Ganjtabesh M (2018) First-spike-based visual categorization using reward-modulated STDP. IEEE Trans Neural Netw Learn Syst 29:6178–6190
Nobukawa S, Aiura H, Yoshida H, Nishimura H, Yamanishi T (2018a) Temporal fluctuation in spontaneous activity of a spiking neural network with long-tailed synaptic weights. In: Proceedings of 2018 international symposium on nonlinear theory and its applications (NOLTA 2018), IEICE, pp 375–378
Nobukawa S, Nishimura H, Yamanishi T (2018b) Skewed and long-tailed distributions of spiking activity in coupled network modules with log-normal synaptic weight distribution. In: International conference on neural information processing, Springer, pp 535–544
Nobukawa S, Kikuchi M, Takahashi T (2019a) Changes in functional connectivity dynamics with aging: a dynamical phase synchronization approach. NeuroImage 188:357–368
Nobukawa S, Nishimura H, Yamanishi T (2019b) Pattern classification by spiking neural networks combining self-organized and reward-related spike-timing-dependent plasticity. J Artif Intell Soft Comput Res 9(4):283–291
Nobukawa S, Nishimura H, Yamanishi T (2019c) Temporal-specific complexity of spiking patterns in spontaneous activity induced by a dual complex network structure. Sci Rep 9(1):1–12
Nobukawa S, Yamanishi T, Nishimura H, Wada Y, Kikuchi M, Takahashi T (2019d) Atypical temporal-scale-specific fractal changes in Alzheimer’s disease EEG and their relevance to cognitive decline. Cogn Neurodyn 13(1):1–11
Nobukawa S, Yamanishi T, Kasakawa S, Nishimura H, Kikuchi M, Takahashi T (2020) Classification methods based on complexity and synchronization of electroencephalography signals in Alzheimer’s disease. Front Psychiatry 11:255
Nurujjaman M, Narayanan R, Iyengar AS (2009) Comparative study of nonlinear properties of EEG signals of normal persons and epileptic patients. Nonlinear Biomed Phys 3(1):6
Okazaki R, Takahashi T, Ueno K, Takahashi K, Ishitobi M, Kikuchi M, Higashima M, Wada Y (2015) Changes in EEG complexity with electroconvulsive therapy in a patient with autism spectrum disorders: a multiscale entropy approach. Front Hum Neurosci 9:106
Oprea L, Pack CC, Khadra A (2020) Machine classification of spatiotemporal patterns: automated parameter search in a rebounding spiking network. Cogn Neurodyn 14:267–280
Rabinovich MI, Varona P, Selverston AI, Abarbanel HD (2006) Dynamical principles in neuroscience. Rev Mod Phys 78(4):1213
Sakata S, Harris KD (2009) Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron 64(3):404–418
Samura T, Ikegaya Y, Sato YD (2015) A neural network model of reliably optimized spike transmission. Cogn Neurodyn 9(3):265–277
Schreiber T, Schmitz A (1996) Improved surrogate data for nonlinearity tests. Phys Rev Lett 77(4):635
Stam CJ (2005) Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol 116(10):2266–2301
Takahashi T (2013) Complexity of spontaneous brain activity in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry 45:258–266
Takahashi T, Cho RY, Mizuno T, Kikuchi M, Murata T, Takahashi K, Wada Y (2010) Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage 51(1):173–182
Takahashi T, Yoshimura Y, Hiraishi H, Hasegawa C, Munesue T, Higashida H, Minabe Y, Kikuchi M (2016) Enhanced brain signal variability in children with autism spectrum disorder during early childhood. Hum Brain Mapp 37(3):1038–1050
Tavanaei A, Kirby Z, Maida AS (2018a) Training spiking ConvNets by STDP and gradient descent. In: 2018 international joint conference on neural networks (IJCNN), IEEE, pp 1–8
Tavanaei A, Masquelier T, Maida A (2018b) Representation learning using event-based STDP. Neural Netw 105:294–303
Teplan M et al (2002) Fundamentals of EEG measurement. Meas Sci Rev 2(2):1–11
Teramae J, Tsubo Y, Fukai T (2012) Optimal spike-based communication in excitable networks with strong-sparse and weak-dense links. Sci Rep 2:1–6
Tetko IV, Villa AE (2001) A pattern grouping algorithm for analysis of spatiotemporal patterns in neuronal spike trains. 2. Application to simultaneous single unit recordings. J Neurosci Methods 105(1):15–24
Theiler J, Eubank S, Longtin A, Galdrikian B, Doyne Farmer J (1992) Testing for nonlinearity in time series: the method of surrogate data. Physica D 58:77–94
Uthayakumar R, Easwaramoorthy D (2013) Epileptic seizure detection in EEG signals using multifractal analysis and wavelet transform. Fractals 21(02):1350011
Van de Ville D, Britz J, Michel CM (2010) EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proc Nat Acad Sci 107(42):18179–18184
Vogels TP, Abbott LF (2005) Signal propagation and logic gating in networks of integrate-and-fire neurons. J Neurosci 25(46):10786–10795
Wendt H, Abry P (2007) Multifractality tests using bootstrapped wavelet leaders. IEEE Trans Signal Process 55(10):4811–4820
Wu Y, Deng L, Li G, Zhu J, Shi L (2018) Spatio-temporal backpropagation for training high-performance spiking neural networks. Front Neurosci 12:331
Yang AC, Tsai SJ (2013) Is mental illness complex? From behavior to brain. Prog Neuropsychopharmacol Biol Psychiatry 45:253–257
Zhang J, Cheng W, Liu Z, Zhang K, Lei X, Yao Y, Becker B, Liu Y, Kendrick KM, Lu G et al (2016) Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain 139(8):2307–2321
Acknowledgements
This work was supported by JSPS KAKENHI for Early-Career Scientists Grant Number 18K18124.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nobukawa, S., Wagatsuma, N. & Nishimura, H. Deterministic characteristics of spontaneous activity detected by multi-fractal analysis in a spiking neural network with long-tailed distributions of synaptic weights. Cogn Neurodyn 14, 829–836 (2020). https://doi.org/10.1007/s11571-020-09605-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-020-09605-6