Abstract
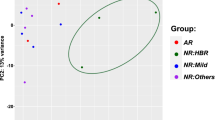
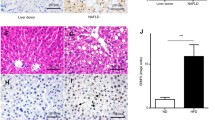
The unfolded protein response (UPR) is an adaptive response that is implicated in multiple metabolic pathologies, including hepatic steatosis. In the present study, we analyzed publicly available RNAseq data to explore how the execution of the UPR is orchestrated in specimens that exhibit hepatocyte ballooning, a landmark feature of steatosis. By focusing on a panel of well-established UPR genes, we assessed how the UPR is coordinated with the whole transcriptome in specimens with or without hepatocyte ballooning. Our analyses showed that neither average levels nor correlation in expression between major UPR genes such as HSPA5 (BiP/GRP78), HSP90b1 (GRP94), or DDIT3 (CHOP) is altered in different groups. However, a panel of transcripts depending on the stringency of the analysis ranged from 16 to 372 lost its coordination with HSPA5, the major UPR chaperone, when hepatocyte ballooning occurred. In 13 genes, the majority of which is associated with metabolic processes, and the coordination with the HSPA5 was reversed from positive to negative in livers with ballooning hepatocytes. In order to examine if during ballooning, UPR genes abolish established and acquire novel functionalities, we performed gene ontology analyses. These studies showed that among the various UPR genes interrogated, only DDIT3 was not associated with conventional functions linked to endoplasmic reticulum stress during ballooning, while HSPA90b1 exhibited the highest function retention between the specimens with or without ballooning. Our results challenge conventional notions on the impact of specific genes in disease and suggest that besides abundance, the mode of coordination of UPR may be more important for disease development.





Similar content being viewed by others
References
Back SH, Kaufman RJ (2012) Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 81:767–793
Barone MV, Crozat A, Tabaee A, Philipson L, Ron D (1994) CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G(1)/S arrest. Genes Dev 8:453–464
Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, Pramoonjago P, Simmons W, Scruggs H, Rosenbaum N, Wilkinson T, Toms P, Argo CK, al-Osaimi AMS, Redick JA (2010) Hepatocellular ballooning in NASH. J Hepatol 53(4):719–723. https://doi.org/10.1016/j.jhep.2010.04.031
Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ (2010) CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 299:G236–G243
Chen WT, Zhu G, Pfaffenbach K, Kanel G, Stiles B, Lee AS (2014a) GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene. 33(42):4997–5005
Chen WT, Tseng CC, Pfaffenbach K, Kanel G, Luo B, Stiles BL, Lee AS (2014b) Liver-specific knockout of GRP94 in mice disrupts cell adhesion, activates liver progenitor cells, and accelerates liver tumorigenesis. Hepatology. 59(3):947–957
Cheretis C, Dietrich F, Chatzistamou I, Politi K, Angelidou E, Kiaris H, Mkrtchian S, Koutselini H (2006 Oct) Expression of ERp29, an endoplasmic reticulum secretion factor in basal-cell carcinoma. Am J Dermatopathol 28(5):410–412
Eden E, Lipson D, Yogev S, Yakhini Z (2007) Discovering motifs in ranked lists of DNA sequences. PLoS Comput Biol 3(3):e39. https://doi.org/10.1371/journal.pcbi.0030039
Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z (2009) GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10:48. https://doi.org/10.1186/1471-2105-10-48
Fornace AJ, Nebert DW, Hollander MC, Luethy JD, Papathanasiou M, Fargnoli J, Holbrook NJ (1989) Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol 9:4196–4203
Frakes AE, Dillin A (2017) The UPRER: sensor and coordinator of organismal homeostasis. Mol Cell 66(6):761–771
Fusakio ME, Willy JA, Wang Y, Mirek ET, al Baghdadi RJ, Adams CM, Anthony TG, Wek RC (2016) Transcription factor ATF4 directs basal and Joshi-Tope G, Vastrik I, Gopinath GR, Matthews L, Schmidt E, Gillespie M, D’Eustachio P, Jassal B, Lewis S, Wu G, Birney E, Stein L. The genome knowledgebase: a resource for biologists and bioinformaticists. Cold Spring Harb Symp Quant Biol.68:237-43metabolism in the liver. Mol Biol Cell 27(9):1536–1551. https://doi.org/10.1091/mbc.E16-01-0039
Gardner BM, Pincus D, Gotthardt K, Gallagher CM (2013) Walter P Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5(3):a013169
Guérin R, Arseneault G, Dumont S, Rokeach LA (2008) Calnexin is involved in apoptosis induced by endoplasmic reticulum stress in the fission yeast. Mol Biol Cell 19(10):4404–4420. https://doi.org/10.1091/mbc.E08-02-0188
Han J, Kaufman RJ (2016) The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res 57(8):1329–1338
Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15:481–490
Havighorst A, Zhang Y, Farmaki E, Kaza V, Chatzistamou I, Kiaris H (2019) Differential regulation of the unfolded protein response in outbred deer mice and susceptibility to metabolic disease. Dis Model Mech 12(2):dmm037242. https://doi.org/10.1242/dmm.037242
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13(8):477–491
Hetz C, Chevet E, Harding HP (2013) Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12(9):703–719
Hoang SA, Oseini A, Feaver RE, Cole B, Asgharpour A, Vincent R, Siddiqui M, Lawson MJ, Day NC, Taylor JM, Wamhoff BR, Mirshahi F, Contos MJ, Idowu M, Sanyal AJ (2019) Distinct molecular profiles of nonalcoholic fatty liver disease. Sci Rep 9(1):12541
Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS (2011) Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 54(1):229–239
Joshi-Tope G, Vastrik I, Gopinath GR, Matthews L, Schmidt E, Gillespie M, D’Eustachio P, Jassal B, Lewis S, Wu G, Birney E, Stein L The genome knowledgebase: a resource for biologists and bioinformaticists. Cold Spring Harb Symp Quant Biol 68:237–243
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F (2009) J Clin Invest. 2009 May;119(5):1201-15. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119(5):1201–1215
Lackner C, Gogg-Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H (2008) Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol 48(5):821–828. https://doi.org/10.1016/j.jhep.2008.01.026.Epub2008Feb 22.
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459. https://doi.org/10.1128/mcb.23.21.7448-7459.2003
Luo S, Mao C, Lee B, Lee AS (2006) GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 26(15):5688–5697. https://doi.org/10.1128/MCB.00779-06
Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han JS, Mauer AS, Yong J, Kaufman RJ (2013) C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem 288:18624–18642
Mast SW, Diekman K, Karaveg K, Davis A, Sifers RN, Moremen KW (2005) Human EDEM2, a novel homolog of family 47 glycosidases, is involved in ER-associated degradation of glycoproteins. Glycobiology 15:421–436
Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD (2008) Non-invasive means of measuring hepatic fat content. World J Gastroenterol 14(22):3476–3483
Mihailidou C, Papazian I, Papavassiliou AG, Kiaris H (2010) CHOP-dependent regulation of p21/waf1 during ER stress. Cell Physiol Biochem 25:761–766
Nassir F, Rector RS, Hammoud GM, Ibdah JA (2015) Pathogenesis and prevention of hepatic steatosis. Gastroenterol Hepatol (N Y) 11(3):167–175
Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M (2002) Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109:525–532
Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ (2010) Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab 298:E1027–E1035
Ramji D, Foka P (2002) CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365:561–575
Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ (2008) UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15(6):829–840
Song MJ, Malhi H (2019) The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol Ther 13:107401. https://doi.org/10.1016/j.pharmthera.2019.107401
Song BB, Scheuner D, Ron D, Pennathur S, Kaufman RJ (2008) Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Investig 118:3378–3389
Tang Q et al (2019) Cell Prolif e12706
van Dam S, Võsa U, van der Graaf A, Franke L, de Magalhães JP (2018) Gene co-expression analysis for functional classification and gene-disease predictions. Brief Bioinform 19(4):575–592. https://doi.org/10.1093/bib/bbw139
Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, Mori K (2010) Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell 21(17):2975–2986
Zhang Y, Lucius MD, Altomare D, Havighorst A, Farmaki E, Chatzistamou I, Shtutman M, Kiaris H (2019 Sep) Coordination analysis of gene expression points to the relative impact of different regulators during endoplasmic reticulum stress. DNA Cell Biol 38(9):969–981. https://doi.org/10.1089/dna.2019.491
Zhu G, Lee AS (2015) Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol 230(7):1413–1420
Funding
This study was supported by NSF (Award Number: 1736150).
Author information
Authors and Affiliations
Contributions
YZ performed the analyses, interpreted results, and edited and approved MS; IC interpreted the results and edited and approved MS; and HK analyzed the data, interpreted the results, and drafted and approved MS.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Ethics approval and consent to participate
N/A
Consent for publication
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Chatzistamou, I. & Kiaris, H. Coordination of the unfolded protein response during hepatic steatosis identifies CHOP as a specific regulator of hepatocyte ballooning. Cell Stress and Chaperones 25, 969–978 (2020). https://doi.org/10.1007/s12192-020-01132-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-020-01132-x