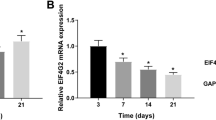
Abstract
Chronic intake of fluoride, existing in the environment, may cause endemic fluorosis, which is characterized by the occurrence of skeletal and dental fluorosis. However, the pathogenesis of fluorosis has not yet been elucidated. Abnormal osteoblast proliferation and activation have a pivotal role in bone turnover disorders which are linked to skeletal fluorosis. MicroRNAs are involved in fundamental cellular processes, including cell proliferation. Based on our previous study, population study and in vitro experiments were designed to understand the effect of miR-122-5p on osteoblast activation in skeletal fluorosis through targeting cyclin-dependent kinase 4 (CDK4). In human populations with coal-burning type fluoride exposure, the results showed that miR-122-5p was downregulated but CDK4 expression was upregulated and miR-122-5p was negatively correlated with CDK4 expression. Furthermore, in human osteoblasts treated with sodium fluoride, we demonstrated that miR-122-5p mediated osteoblast activation of skeletal fluorosis via upregulation of the CDK4 protein. In support of this, dual-luciferase reporter assay showed that miR-122-5p modulated CDK4 protein levels by targeting its 3′-untranslated region. These findings show, for the first time, that miR-122-5p may be involved in the cause and development of skeletal fluorosis by targeting CDK4.







Similar content being viewed by others
References
Singh G, Kumari B, Sinam G, Kriti, Kumar N, Mallick S (2018) Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective-a review. Environ Pollut 239(8):95–108. https://doi.org/10.1016/j.envpol.2018.04.002
Perumal E, Vanaja P, Govindarajan V et al (2013) A brief review on experimental fluorosis. Toxicol Lett 223(2):236–251. https://doi.org/10.1016/j.toxlet.2013.09.005
Dhar V, Bhatnagar M (2009) Physiology and toxicity of fluoride. Indian J Dent Res 20(3):350–355. https://doi.org/10.4103/0970-9290.57379
Luo K, Li L, Zhang S (2011) Coal-burning roasted corn and chili as the cause of dental fluorosis for children in southwestern China. J Hazard Mater 185(2):1340–1347. https://doi.org/10.1016/j.jhazmat.2010.10.052
Maciej JK, Simon F, Beil T, Riedel C (2016) Deterioration of teeth and alveolar bone loss due to chronic environmental high-level fluoride and low calcium exposure. Clin Oral Investig 20(9):2361–2370. https://doi.org/10.1007/s00784-016-1727-1
Li L, Luo KL (2012) The pollution control of fluorine and arsenic in roastedcorn in “coal-burning” fluorosis area Yunnan. China J Hazard Mater 229–230(9):57–65. https://doi.org/10.1016/j.jhazmat.2012.05.067
Jun L, Sheng Y, Ming JL et al (2018) Association of dietary carotenoids intake with skeletal fluorosis in the coal-burning fluorosis area of Guizhou Province. Biomed Environ Sci 31(6):438–447. https://doi.org/10.3967/bes2018.057
Pei JR, Li BY, Gao YH, Wei Y, Zhou L, Yao H, Wang J, Sun D (2014) Fluoride decreased osteoclastic bone resorption through the inhibition of NFATc1 gene expression. Environ Toxicol 29(5):588–595. https://doi.org/10.1002/tox.21784
Wang Q, Cui KP, Xu YY, Gao YL, Zhao J, Li DS, Li XL, Huang HJ (2014) Coal-burning endemic fluorosis is associated with reduced activity in antioxidative enzymes and Cu/Zn-SOD gene expression. Environ Geochem Health 36(1):107–115. https://doi.org/10.1007/s10653-013-9522-2
Yang C, Wang Y, Xu H et al (2017) Treatment and prevention of skeletal fluorosis. Biomed Environ Sci 30(2):147–149. https://doi.org/10.3967/bes2017.020
Wang Z, Yang X, Yang S et al (2011) Sodium fluoride suppress proliferation and induce apoptosis through decreased insulin-like growth factor-I expression and oxidative stress in primary cultured mouse osteoblasts. Arch Toxicol 85:1407–1417. https://doi.org/10.1007/s00204-011-0697-y
Qu WJ, Zhong DB, Wu PF, Wang JF, Han B (2008) Sodium fluoride modulates caprine osteoblast proliferation and differentiation. J Bone Miner Metab 26(4):328–334. https://doi.org/10.1007/s00774-007-0832-2
Pan L, Shi X, Liu S, Guo X, Zhao M, Cai R, Sun G (2014) Fluoride promotes osteoblastic differentiation through canonical Wnt/β-catenin signaling pathway. Toxicol Lett 225(1):34–42. https://doi.org/10.1016/j.toxlet.2013.11.029
Huo L, Liu K, Pei J, Yang Y, Ye Y, Liu Y, Sun J, Han H, Xu W, Gao Y (2013) Fluoride promotes viability and differentiation of osteoblast-like Saos-2 cells via BMP/Smads signaling pathway. Biol Trace Elem Res 155(1):142–149. https://doi.org/10.1007/s12011-013-9770-0
Topacio BR, Zatulovskiy E, Cristea S et al (2019) Cyclin D-Cdk4,6 drives cell-cycle progression via the retinoblastoma protein’s C-terminal helix. Mol Cell 16;74(4):758–770.e4. https://doi.org/10.1016/j.molcel
Bošković A, Rando OJ (2018) Transgenerational epigenetic inheritance. Annu Rev Genet 52:21–41. https://doi.org/10.1146/annurev-genet-120417-031404
Martos SN, Tang WY, Wang ZB (2015) Elusive inheritance: transgenerational effects and epigenetic inheritance in human environmental disease. Prog Biophys Mol Biol 118(1–2):44–54. https://doi.org/10.1016/j.pbiomolbio.2015.02.011
Wang HS, Lou D, Wang ZB (2019) Crosstalk of genetic variants, allele-specific DNA methylation, and environmental factors for complex disease risk. Front Genet 9:695. https://doi.org/10.3389/fgene.2018.00695
Morales S, Monzo M, Navarro A (2017) Epigenetic regulation mechanisms of microRNA expression. Biomol Concepts 8(5–6):203–212. https://doi.org/10.1515/bmc-2017-0024
Wu HJ, Liu Y, Shu XO et al (2016) miR-374a suppresses lung adenocarcinoma cell proliferation and invasion by targeting TGFA gene expression. Carcinogenesis 37(6):567–575. https://doi.org/10.1093/carcin/bgw038
Li Z, Lu J, Zeng G et al (2019) miR-129-5p inhibits liver cancer growth by targeting calcium calmodulin-dependent protein kinase IV (CAMK4). Cell Death Dis 10(11):789. https://doi.org/10.1038/s41419-019-1923-4
Hsu HH, Kuo WW, Shih HN, Cheng SF, Yang CK, Chen MC, Tu CC, Viswanadha VP, Liao PH, Huang CY (2019) FOXC1 regulation of miR-31-5p confers oxaliplatin resistance by targeting LATS2 in colorectal cancer. Cancers (Basel) 11(10):1576. https://doi.org/10.3390/cancers11101576
Tang Y, Meng X, Yu X et al (2019) Inhibition of microRNA-875-5p promotes radioiodine uptake in poorly differentiated thyroid carcinoma cells by upregulating sodium–iodide symporter. J Endocrinol Investig. https://doi.org/10.1007/s40618-019-01125-3
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597–610. https://doi.org/10.1038/nrg2843
Herranz H, Cohen SM (2010) MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev 24:1339–1344. https://doi.org/10.1101/gad.1937010
Ming J, Wu SL, You TZ, Wang X, Yu C, Luo P, Zhang A, Pan X (2019) Histone deacetylation in the promoter of p16 is involved in fluoride-induced human osteoblast activation via the inhibition of Sp1 binding. Biol Trace Elem Res 188(2):373–383. https://doi.org/10.1007/s12011-018-1413-z
You TZ, Liao YD, Yan WM et al (2016) Effects of sodium fluoride on histone acetylation and expression of p21 gene in human primary osteoblasts. J Environ Occup Med 33(6):536–541. https://doi.org/10.13213/j.cnki.jeom.2016.16210
Wu S, Yan W, Qiu B, Liao Y, Gu J, Wei S, Zhang A, Pan X (2019) Aberrant methylation-induced dysfunction of p16 is associated with osteoblast activation caused by fluoride. Environ Toxicol 34(1):37–47. https://doi.org/10.1002/tox.22655
Pan X, Yan WM, Qiu B et al (2020) Aberrant DNA methylation of Cyclind-CDK4-p21 is associated with chronic fluoride poisoning. Chem Biol Interact 315:108875. https://doi.org/10.1016/j.cbi.2019
Yan WM, Ming J, You TZ, et al (2018) The effects of sodium fluoride on histone acetylation of CyclinD1/cyclin-dependent kinases 4 gene in human osteoblasts. Chinese Journal of Endemiology (1):13-18. DOI:https://doi.org/10.3760/cma.j.issn.2095-4255.2018.01.004
Wang W, Zhao LJ, Tan YX et al (2012) miR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis 33(5):1113–11uu20. https://doi.org/10.1093/carcin/bgs113
Yin T, Liu MM, Jin RT et al (2019) miR-152-3p modulates hepatic carcinogenesis by targeting cyclin-dependent kinase 8. Pathol Res Pract 215(6):152406. https://doi.org/10.1016/j.prp.2019.03.034
Zhu KG, Lin L, Zhang JL et al (2016) miR-29b suppresses the proliferation and migration of osteosarcoma cells by targeting CDK6. Protein Cell 7(6):434–444. https://doi.org/10.1007/s13238-016-0277-2
Yang HQ, Wang LX, Tang XL, Bai W (2017) miR-203a suppresses cell proliferation by targeting E2F transcription factor 3 in human gastric cancer. Oncol Lett 14(6):7687–7690. https://doi.org/10.3892/ol.2017.7199
Wang F, Li C, Qin Y, Han X, Gao J, Zhang A, Luo P, Pan X (2019) Analysis of the microRNA profile of coal-burning endemic fluorosis using deep sequencing and bioinformatic approaches. Bull Environ Contam Toxicol 103(1):56–63. https://doi.org/10.1007/s00128-019-02660-8
Ministry of Health of the People’s Republic of China (2015) Determination of fluoride in urine-ion selective electrode method (WS/T 89–2015), Issued in 2015
Ministry of Health of the People’s Republic of China (2005) The normal concentration of urinary fluoride of population (WS/T 256–2005), Issued in 2005
Chen S, Li B, Lin S, Huang Y, Zhao X, Zhang M, Xia Y, Fang X, Wang J, Hwang SA, Yu S (2013) Change of urinary fluoride and bone metabolism indicators in the endemic fluorosis areas of southern China after supplying low fluoride public water. BMC Public Health 13:156–166. https://doi.org/10.1186/1471-2458-13-156
Kurdi MS (2016) Chronic fluorosis: the disease and its anaesthetic implications. Indian J Anaesth 60(3):157–162. https://doi.org/10.4103/0019-5049.177867
Pramanik S, Saha D (2017) The genetic influence in fluorosis. Environ Toxicol Pharmacol 56:157–162. https://doi.org/10.1016/j.etap.2017.09.008
Wei S, Jiang X, Qiu B et al (2016) Correlation between urinary fluoride and bone metabolic index of patients with endemic fluorosis induced by coal burning and its reference dose analysis. J Environ Health 33(9):801–803
Song YE, Tan H, Liu KJ et al (2011) Effect of fluoride exposure on bone metabolism indicators ALP, BALP, and BGP. Environ Health Prev Med 16(3):158–163. https://doi.org/10.1007/s12199-010-0181-y
Jiang YT, Yang YM, Wang HG, Darko GM, Sun D, Gao Y (2018) Identification of miR-200c-3p as a major regulator of SaoS2 cells activation induced by fluoride. Chemosphere 199:694–701. https://doi.org/10.1016/j.chemosphere.2018.01.095
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9(2):102–114. https://doi.org/10.1038/nrg2290
Huntzinger E, Izaurralde E (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12(2):99–110. https://doi.org/10.1038/nrg2936
Clément T, Salone V, Rederstorff M (2015) Dual luciferase gene reporter assays to study miRNA function. Methods Mol Biol 1296:187–198. https://doi.org/10.1007/978-1-4939-2547-6_17
Wang Y, Chang WG, Zhang Y, Zhang L, Ding H, Qi H, Xue S, Yu H, Hu L, Liu D, Zhu W, Wang Y, Li P (2019) Circulating miR-22-5p and miR-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. J Cell Physiol 234(4):4778–4786. https://doi.org/10.1002/jcp.27274
Mard SA, Akbari G, Dianat M, Mansouri E (2019) The effect of zinc sulfate on miR-122, miR-34a, atioxidants, biochemical and histopathological parameters following hepatic ischemia/ reperfusion injury in rats. Biol Trace Elem Res 188(2):434–440. https://doi.org/10.1007/s12011-018-1425-8
Meng L, Chen ZM, Jiang Z, Huang T, Hu J, Luo P, Zhang H, Huang M, Huang L, Chen Y, Lu M, Xu AM, Ying S (2020) miR-122-5p suppresses the proliferation, migration, and invasion of gastric cancer cells by targeting LYN. Acta Biochim Biophys Sin 52(1):49–57. https://doi.org/10.1093/abbs/gmz141
Xu Z, Liu G, Zhang M, Zhang Z, Jia Y, Peng L, Zhu Y, Hu J, Huang R, Sun X (2018) miR-122-5p inhibits the proliferation, invasion and growth of bile duct carcinoma cells by targeting ALDOA. Cell Physiol Biochem 48(6):2596–2606. https://doi.org/10.1159/000492702
Liu YH, Liu JL, Wang Z et al (2019) miR-122-5p suppresses cell proliferation, migration and invasion by targeting SATB1 in nasopharyngeal carcinoma. Eur Rev Med Pharmacol Sci 23(2):622–629. https://doi.org/10.26355/eurrev_201901_16876
Raghunath A, Jeyabaskar D, Sundarraj K et al (2016) In silico prediction of microRNAs on fluoride induced sperm toxicity in mice. Food Chem Toxicol 98(Pt,A):34–49. https://doi.org/10.1016/j.fct.2016.03.005
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81660524) and the First-Class Discipline Construction Project in Guizhou Province - Public Health and Preventive Medicine (No. 2017[85]).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study received approval from the Ethical Committee of Guizhou Medical University. All participants involved in this research signed informed consent forms.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• miR-122-5p levels were downregulated, and CDK4 expression was upregulated in the fluoride-exposed population.
• Downregulation of miR-122-5p is involved in fluoride-induced human osteoblast activation by increasing the CDK4 protein expression.
• miR-122-5p regulates CDK4 protein expression by targeting its 3′-UTR.
Electronic Supplementary Material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Li, C., Qin, Y., Ouyang, T. et al. miR-122-5p Mediates Fluoride-Induced Osteoblast Activation by Targeting CDK4. Biol Trace Elem Res 199, 1215–1227 (2021). https://doi.org/10.1007/s12011-020-02239-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02239-z