Abstract
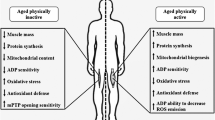
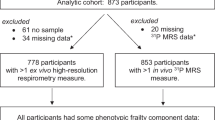
Although a persistent inflammatory state has long been associated with aging and negative health outcomes, the underlying mechanisms remain unclear. Mitochondrial dysfunction has been proposed as a cause of inflammaging, but evidence of an association in humans is lacking. In this study, we analyzed the cross-sectional association between inflammatory biomarkers and mitochondrial oxidative capacity in skeletal muscle, assessed as post-exercise phosphocreatine recovery time constant by phosphorus magnetic resonance spectroscopy, in a population of 669 adults (mean age 67 years) from the Baltimore Longitudinal Study of Aging. We observed that participants with lower mitochondrial oxidative capacity exhibited hallmarks of inflammation, specifically markedly higher levels of interleukin-6 and C-reactive protein, as well as increased erythrocyte sedimentation rate when compared with participants with better oxidative capacity, independent of age and sex. We speculate that this association reflects the observation that products of damaged mitochondria, such as mitochondrial DNA, activate multiple pathways that lead to inflammation. Furthermore, excess production of oxidative species (ROS) by dysfunctional mitochondria could trigger inflammation either directly via NF-κB or through oxidative damage to proteins, lipids, and nucleic acids. Longitudinal studies are necessary to ascertain whether and through which mechanisms mitochondrial dysfunction activate inflammation or whether both these phenomena derive from a common root.


Similar content being viewed by others
References
Adelnia F, Urbanek J, Osawa Y, Shardell M, Brennan NA, Fishbein KW, et al. Moderate-to-vigorous physical activity is associated with higher muscle oxidative capacity in older adults. J Am Geriatr Soc. 2019;67:1695–9.
Arnold D, Matthews P, Radda G. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984;1:307–15.
Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:120–31.
Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB, Health A, et al. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–9.
Choi S, et al. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71:1638–45.
Chung HY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30.
Coen PM, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2012;68:447–55.
Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–8.
Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526:203–10.
De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–72.
Dhanwani R, Takahashi M, Sharma S. Cytosolic sensing of immuno-stimulatory DNA, the enemy within. Curr Opin Immunol. 2018;50:82–7.
Ershler WB. Interleukin-6: a cytokine for gerontolgists. J Am Geriatr Soc. 1993;41:176–81.
Fabbri E, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2014;70:63–70.
Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–22.
Ferrucci L, Zampino M. A mitochondrial root to accelerated ageing and frailty. Nat Rev Endocrinol. 2020;16:133–4.
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–9.
Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science. 2011;333:1109–12.
Guralnik JM, Fried LP, Simonsick EM, Lafferty ME, Kasper JD. The Women’s Health and Aging Study: health and social characteristics of older women with disability: DIANE Publishing; 1995.
Harris TB, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12.
Kuo PL, et al. A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. J Intern Med. 2020;287:373–94.
Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual, vol. 177. Champaign, IL: Human kinetics books; 1988.
López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–18.
Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–84.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Naressi A, Couturier C, Castang I, De Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–86.
Paganini A, Foley J, Meyer R. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272:C501–10.
Pinti M, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging”. Eur J Immunol. 2014;44:1552–62.
Porter C, et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309:E224–32.
Prompers JJ, Wessels B, Kemp GJ, Nicolay K. MITOCHONDRIA: investigation of in vivo muscle mitochondrial function by 31P magnetic resonance spectroscopy. Int J Biochem Cell Biol. 2014;50:67–72.
Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69:2999–3013.
Sastre J, Pallardó FV, de la Asunción García J, Viña J. Mitochondria, oxidative stress and aging. Free Radic Res. 2000;32:189–98.
Semba RD, Moaddel R, Zhang P, Ramsden CE, Ferrucci L. Tetra-linoleoyl cardiolipin depletion plays a major role in the pathogenesis of sarcopenia. Med Hypotheses. 2019;127:142–9.
Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore Longitudinal Study. J Gerontol. 1966;21:575–80.
Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61:654–66.
Taylor D, Styles P, Matthews P, Arnold D, Gadian D, Bore P, et al. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986;3:44–54.
Tschopp J. Mitochondria: sovereign of inflammation? Eur J Immunol. 2011;41:1196–202.
Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140:120–30.
Wang J, Leung K-S, Chow SK-H, Cheung W-H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J Orthop Transl. 2017;10:94–101.
White MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–62.
Zimmermann M, et al. IFNα enhances the production of IL-6 by human neutrophils activated via TLR8. Sci Rep. 2016;6:19674.
Funding
This work was supported by the Intramural Research Program of the National Institute on Aging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences (NIEHS, NC). All participants consented to participate in the study, after receiving detailed descriptions at every visit.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zampino, M., Brennan, N.A., Kuo, PL. et al. Poor mitochondrial health and systemic inflammation? Test of a classic hypothesis in the Baltimore Longitudinal Study of Aging. GeroScience 42, 1175–1182 (2020). https://doi.org/10.1007/s11357-020-00208-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00208-x