Abstract
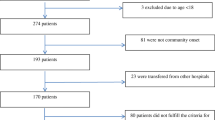
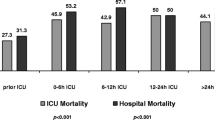
Prescribing antibiotics for febrile patients without proof of bacterial infection contributes to antimicrobial resistance. Lack of clinical response in these patients often leads to antibiotic escalation, although data supporting this strategy are scarce. This study compared outcomes of modifying, withholding, or continuing the same antibiotic regimen for such patients. Febrile or hypothermic stable patients with suspected infection, unresponsive to empiric antibiotic treatment, admitted to one of 15 internal medicine departments in three hospitals during a 5-year study period, were included. Patients with a definitive clinical or microbiological bacterial infection, malignancy, immunodeficiency, altered mental status, or need for mechanical ventilation were excluded. Participants were divided into groups based on treatment strategy determined 72 h after antibiotic initiation: antibiotic modified, withheld or continued. Outcomes measured included in-hospital and 30-day post-discharge-mortality rates, length of hospital stay (LOS) and days of antimicrobial therapy (DOT). A total of 486 patients met the inclusion criteria: 124 in the Antibiotic modified group, 67 in the Antibiotic withheld group and 295 in the Initial antibiotic continued group. Patient characteristics were similar among groups with no differences in mortality rates in-hospital (23% vs. 25% vs. 20%, p = 0.58) and within 30 days after discharge (5% vs. 3% vs. 4%, p = 0.83). Changing antibiotics led to longer LOS (9.0 ± 6.8 vs. 6.2 ± 5.6 days, p = 0.003) and more DOT (8.6 ± 6.0 vs. 3.2 ± 1.0 days, p < 0.001) compared to withholding treatment. Withholding as compared to modifying antibiotics, in febrile patients with no clear evidence of bacterial infection, is a safe strategy associated with decreased LOS and DOT.


Similar content being viewed by others
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services (2019) Antibiotic resistance threats in the United States 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 18 Mar 2019
Barnes SL, Rock C, Harris AD et al (2017) The impact of reducing antibiotics on the transmission of multidrug-resistant organisms. Infect Control Hosp Epidemiol 38:663–669. https://doi.org/10.1017/ice.2017.34
Schechner V, Temkin E, Harbarth S et al (2013) Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev 26(2):289–307. https://doi.org/10.1128/CMR.00001-13
Tamma PD, Avdic E, Li DX et al (2017) Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 177(9):1308–1315. https://doi.org/10.1001/jamainternmed.2017.1938
Lin S, Xiaoyan Z, Yuxiao Z et al (2019) Antibiotic-induced disruption of gut microbiota alters local metabolomes and immune responses. Front Cell Infect Microbiol 9:99. https://doi.org/10.3389/fcimb.2019.00099
Willmann M, Vehreschild MJGT, Biehl LM et al (2019) Distinct impact of antibiotics on the gut microbiome and resistome: a longitudinal multicenter cohort study. BMC Biol 17:76. https://doi.org/10.1186/s12915-019-0692-y
Brown K, Valenta K, Fisman D et al (2015) Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med 175:626–633. https://doi.org/10.1001/jamainternmed.2014.8273
Zilberberg MD, Nathanson BH, Sulham K et al (2016) Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care 20:221. https://doi.org/10.1186/s13054-016-1392-4
Iregui M, Ward S, Sherman G et al (2002) Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122(1):262–268. https://doi.org/10.1378/chest.122.1.262
Shorr AF, Micek ST, Welch EC et al (2011) Inappropriate antibiotic therapy in gram-negative sepsis increases hospital length of stay. Crit Care Med 39:46–51. https://doi.org/10.1097/CCM.0b013e3181fa41a7
Grossman C, Keller N, Bornstein G et al (2016) Factors associated with suitability of empiric antibiotic therapy in hospitalized patients with bloodstream infections. Chemother 9(3):159–163. https://doi.org/10.1080/1120009X.2016.1182770
Zilberberg MD, Shorr AF, Micek ST et al (2008) Antimicrobial therapy escalation and hospital mortality among patients with HCAP: a single center experience. Chest 134:963–968. https://doi.org/10.1378/chest.08-0842
Kumar A, Ellis P, Arabi Y et al (2009) Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136(5):1237–1248. https://doi.org/10.1378/chest.09-0087
Alvarez-Lerma F (1996) Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med 22(5):387–394. https://doi.org/10.1007/BF01712153
Pardo J, Klinker KP, Boger SJ et al (2014) Time to positivity of blood cultures supports antibiotic de-escalation at 48 hours. Ann Pharmacother 48:33–40. https://doi.org/10.1177/1060028013511229
Seymour CW, Liu VX, Iwashyna TJ et al (2016) Assessment of clinical criteria for Sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):762–774. https://doi.org/10.1001/jama.2016.0288
Barlam TF, Cosgrove SE, Abbo LM et al (2016) Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–e77. https://doi.org/10.1093/cid/ciw118
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Rodrigues CMC, Groves H (2018) Acquired pneumonia in children: the challenges of microbiological diagnosis. J Clin Microbiol 56(3):e01318–e01317. https://doi.org/10.1128/JCM.01318-17
Chanu R (2017) Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis 4(1):249. https://doi.org/10.1093/ofid/ofw249
Christ-Crain M, Jaccard-Stolz D, Bingisser R et al (2004) Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 363(9409):600–607. https://doi.org/10.1016/s0140-6736(04)15591-8
Carratala J, Garcia-Vidal C, Ortega L et al (2012) Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med 172:922–928. https://doi.org/10.1001/archinternmed.2012.1690
Fine MJ, Stone RA, Lave JR et al (2003) Implementation of an evidence-based guideline to reduce duration of intravenous antibiotic therapy and length of stay for patients hospitalized with community-acquired pneumonia: a randomized controlled trial. Am J Med 15:343–351. https://doi.org/10.1016/s0002-9343(03)00395-4
Avdic E, Cushinotto LA, Hughes AH et al (2012) Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 54:1581–1587. https://doi.org/10.1093/cid/cis242
Hauck LD, Adler LM, Mulla ZD (2004) Clinical pathway care improves outcomes among patients hospitalized for community-acquired pneumonia. Ann Epidemiol 14:669–675. https://doi.org/10.1016/j.annepidem.2004.01.003
Ibrahim EH, Ward S, Sherman G et al (2001) Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med 29:1109–1115. https://doi.org/10.1097/00003246-200106000-00003
Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services (2019) Core elements of hospital antibiotic stewardship programs. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed 25 Mar 2019
Thom AT, Tamma PD, Harris AD et al (2019) Impact of a prescriber-driven antibiotic time-out on antibiotic use in hospitalized patients. Clin Infect Dis 68(9):1581–1584. https://doi.org/10.1093/cid/ciy852
Lee TC, Frenette C, Jayaraman D et al (2014) Antibiotic self-stewardship: trainee-led structured antibiotic time-outs to improve antimicrobial use. Ann Intern Med 161(10):s53–s58. https://doi.org/10.7326/M13-3016
Lesprit P, Landelle C, Girou E et al (2010) Reassessment of intravenous antibiotic therapy using a reminder or direct counselling. J Antimicrob Chemother 65:789–795. https://doi.org/10.1093/jac/dkq018
Acknowledgements
We would like to thank Faye Schreiber for editorial assistance and Navah Jelin for statistical consultation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval, consent to participate and consent for publication
The study was approved by Institutional Helsinki Committees at each of the two participating medical centres (approval numbers: RMC-0814-17 and MMC-0219-18). Informed consents to participate and for publication were not required due to the retrospective study design.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Mudrik-Zohar, H., Nissan, R., Stein, G.Y. et al. Antibiotic modification versus withhold in febrile patients without evidence of bacterial infection, unresponsive to initial empiric regimen: a multicentre retrospective study conducted in Israel. Eur J Clin Microbiol Infect Dis 39, 2027–2035 (2020). https://doi.org/10.1007/s10096-020-03957-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-03957-x