Towards a Redefinition of Cognitive Frailty
Abstract
Background:
The progressive aging of the population will dramatically increase the burden of dementia related to Alzheimer’s disease (AD) and other neurodegenerative disorders in the future. Because of the absence of drugs that can modify the neuropathological substrate of AD, research is focusing on the application of preemptive and disease-modifying strategies in the pre-symptomatic period of the disease. In this perspective, the identification of people with cognitive frailty (CF), i.e., those individuals with higher risk of developing dementia, on solid pathophysiological bases and with clear operational clinical criteria is of paramount importance.
Objective/Methods:
This hypothesis paper reviews the current definitions of CF, presents and discusses some of their limitations, and proposes a framework for updating and improving the conceptual and operational definition of the CF construct.
Results:
The potential for reversibility of CF should be supported by the assessment of amyloid, tau, and neuronal damage biomarkers, especially in younger patients. Physical and cognitive components of frailty should be considered as separate entities, instead of part of a single macro-phenotype. CF should not be limited to the geriatric population, because trajectories of amyloid accumulation are supposed to start earlier than 65 years in AD. Operational criteria are needed to standardize assessment of CF.
Conclusion:
Based on the limitations of current CF definitions, we propose a revised one according to a multidimensional subtyping. This new definition might help stratifying CF patients for future trials to explore new lifestyle interventions or disease-modifying pharmacological strategies for AD and dementia.
INTRODUCTION
Life expectancy has dramatically increased in the last decades thanks to improved nutrition, hygiene, living conditions, and widely available medical care. The proportion of elderly people, and in particular the oldest old, i.e., individuals older than 80 years, is growing fastest than any other age group and is expected to triple between 2015 and 2050 [1]. Alongside, the increased longevity carries several implications for the quality of life. Aging has been conceptualized as the progressive and overall physiological decline of an organism to produce adaptive responses to stressors due to accumulation of pathologies in different tissues [2, 3]. Older individuals therefore become more susceptible to adverse outcomes [4] and more vulnerable to mild endogenous and/or exogenous stressors, after which returning to baseline condition may be difficult [5]. As a result, aging represents the major risk factor for highly prevalent chronic conditions including cancer and cardiovascular disease [6]. Since aging is the most important and non-modifiable risk factor for cognitive deterioration, the burden of Alzheimer’s disease (AD) and other neurodegenerative disorders is expected to grow dramatically in the future. Reliable forecasts predict approximately 74.7 million dementia cases in the world by 2030, reaching 131.5 million by 2050 [7]. In this scenario, growing attention is being devoted to preventive and health promotion strategies to reverse, or at least improve, the age-related functional decline, reducing the duration of late-life morbidity and disability [8, 9], and increasing successful aging, which is characterized by life satisfaction, freedom from disability, mastery, active engagement with life, independence, social well-being, high cognitive and physical functional capacity [10].
Current pharmacological treatments for AD (i.e., cholinesterase inhibitors and memantine) are symptomatic ones with limited efficacy and considerable side effects, but do not increase life expectancy or slow progression to more severe stages of dementia [11]. Some potentially disease-modifying treatments have been tested to reduce amyloid deposition in AD, but no significant clinical effect has been achieved, and no disease-modifying drugs have been approved yet [12, 13].
According to the National Institute on Aging (NIA) and the Alzheimer’s Association (AA) criteria for preclinical AD diagnosis [14–16], research into future disease-modifying treatments for AD should target both amyloid deposition and tau pathology and recruit patients with early and preclinical disease stages [17]. Pharmacological and non-pharmacological interventions for preclinical stages of AD may target cognitively unimpaired individuals who carry high AD risk on the basis of biomarkers and/or genetic background to influence the disease trajectory [18, 19]. Clinical trials in cognitively unimpaired individuals at risk for AD may document a disease-modifying activity more effectively than those in clinically affected people, even at early disease stage [15, 20, 21]. The window of therapeutic and preventive opportunity for AD may be represented by the very long pre-symptomatic period, and there is concern that promising drugs failed in trials because they were given too late [22].
From this perspective, identifying people with cognitive frailty (CF), i.e., those individuals who carry a higher risk of developing AD-related or other types of dementia on solid pathophysiological bases and with clear operational clinical criteria is of paramount importance. In the community setting, the prevalence of CF was reported to be 1.0–1.8% and to increase in the clinical setting [23]. In the present hypothesis paper, we will review the current definitions of CF, present and discuss some of their limitations, and propose a framework for updating and improving the conceptual and operational definition of the CF construct.
THE CONCEPT OF FRAILTY IN THE ELDERLY: DEFINITIONS AND MODELS
Preventive and health promotion strategies need specific indicators of early functional decline to be effective. In this context, the concept of frailty was developed and reached broad consensus in clinical and research settings [24–28]. First reported in the geriatric medicine literature in the 1950s and 1960s, the term frailty was further developed in the following decades, but its formal operational definition is more recent [29]. We will briefly review the main and most recent definitions of frailty.
The Fried’s phenotype model conceptualized frailty as a geriatric syndrome resulting from cumulative declines across multiple physiological systems and causing vulnerability to adverse outcomes such as falls, disability, institutionalization, and death [29]. The frailty phenotype model involved exclusively the physical domain upon five criteria, i.e., unintentional weight loss, self-reported exhaustion, slowness of gait speed, reduced hand grip strength, and physical activity, with no, 1–2, or ≥3 criteria defining robustness, pre-frailty, and frailty, respectively [29].
The more recent Rockwood’s deficit accumulation model considered multiple domains including medical, functional, and psychosocial aspects, but was also focused on measures of physical performance and the presence of functional deficits [30].
Aware of the limitations of models centered on physical domains only, the Gobbens’ model of frailty was based on a holistic view that included nutrition and cognition in addition to physical features with the elderly positioned on a dynamic continuum between a non-frail and a very frail status, which can be modified through preventive and therapeutic interventions [24, 31].
The biopsychosocial model approach to frailty combines the physical and psychosocial domains, expands the construct of frailty towards social sciences, and includes cognitive, emotional, motivational, and social features. According to this model, the risk of losing social and general resources, activities, or abilities that are important for fulfilling one or more basic psychosocial needs during the life span may increase the risk of developing AD or other neurodegenerative dementias and should be a target for the earliest intervention [32].
According to these models, frailty is a multidimensional construct that can be divided into three major phenotypes, i.e., physical frailty [29], CF [33], and psychosocial frailty [25, 34].
THE CONCEPT OF COGNITIVE FRAILTY: DEFINITIONS AND STATE OF THE ART
The concept of CF has undergone several changes over the years, and we will briefly review its definitions (Table 1).
Table 1
Cognitive frailty: current definitions and operational criteria
| Definition/criteria | |
| CF/Predementia syndrome [35] | State of cognitive vulnerability exposed to vascular risk factors with an increased likelihood of progression to overt dementia |
| IANA-IAGG CF [36] | Heterogeneous clinical manifestation characterized by the simultaneous presence of physical frailty and MCI (CDR score = 0.5); exclusion of concurrent AD-related or other dementia; potentially reversible |
| (Potentially) reversible CF [33] | Potentially reversible CF: MCI (CDR score = 0.5) plus physical/pre-physical frailty |
| Reversible CF: SCD and/or positive fluid and imaging biomarkers of amyloid accumulation and neurodegeneration plus physical/pre-physical frailty |
AD, Alzheimer’s disease; CDR, Clinical Dementia Rating scale; CF, cognitive frailty; IAGG, International Association of Gerontology and Geriatrics; IANA, International Academy on Nutrition and Aging; MCI, mild cognitive impairment; SCD, subjective cognitive decline.
CF was first used in a 2006 review article to indicate a state of cognitive vulnerability in patients with mild cognitive impairment (MCI) exposed to vascular risk factors with an increased likelihood of progression to overt dementia [35].
The International Academy on Nutrition and Aging (IANA) and the International Association of Gerontology and Geriatrics (IAGG) reached a consensus in 2013 on the first definition of CF, which was conceptualized as a condition characterized by a heterogeneous clinical manifestation characterized by the simultaneous presence of physical frailty and MCI (i.e., Clinical Dementia Rating, CDR score = 0.5) without a concurrent diagnosis of AD-related or other types of dementia [36]. According to the IANA-IAGG definition, CF represents a state of reduced cognitive reserve, different from physiological brain aging and potentially reversible, occurring at an intermediate stage between age-related cognitive changes and neurodegenerative diseases [37–40]. A psychological component of CF may concur to increase the vulnerability of the individual to stressors [36]. Despite physical frailty and poor cognition appear to be associated in the IANA-IAGG definition, the causal link between these two entities is unclear.
Two subtypes of CF were later proposed by Ruan and coworkers, i.e., potentially reversible CF and reversible CF, with the former being represented by MCI (CDR score = 0.5) and the latter by subjective cognitive decline (SCD) and/or positive fluid and imaging biomarkers of amyloid accumulation and neurodegeneration, both resulting from physical or pre-physical frailty [33]. According to the SCD Initiative Working Group, SCD is characterized by subjective experience of worsening in cognition, typically in the memory domain, in the absence of objective cognitive deficits at formal neuropsychological evaluation (CDR score = 0) [41]. The potential reversibility of CF suggests it may be an important target for the prevention of dependency and other negative outcomes in older age.
There is increasing awareness that prevention of dementia is important, given the absence of disease-modifying treatments [12, 13]. CF is a topic of great interest in this context, but the current scenario is still far from being clear. Moreover, most studies consider CF as a geriatric condition, but the view of its potential reversibility [33] underscores the importance of neuropsychological rehabilitation. The aim of this hypothesis article is to address core issues in CF research and suggest a roadmap towards a CF redefinition that includes the geriatric, as well as the neurological and neuropsychological perspectives.
THE POTENTIAL FOR REVERSIBILITY OF COGNITIVE FRAILTY
After the proposal of Ruan and collaborators [33], research focused on the potential reversibility of CF [42]. MCI was originally defined as a transitional state between normal aging and dementia, mainly AD-related one, and characterized by mild cognitive disturbances without functional impairment [43]. According to this definition, trajectories of progression might include AD-related dementia for amnestic MCI [14, 44], and other neurodegenerative dementias (e.g., dementia with Lewy bodies, frontotemporal dementia) for non-amnestic MCI [45–47], with a higher yearly risk (5–10%) than the general population (1–2%) of conversion to dementia [48, 49].
Studies that focused on the neuropsychological predictors of MCI conversion to dementia showed that MCI can remain clinically stable for decades [50] or revert to normal cognition at follow-up, especially when a single cognitive domain is involved [51, 52]. However, MCI cases who revert to normal cognition have been demonstrated to carry a high risk of progressing to dementia later on [49]. Based on these reports, the data on MCI reversion rate to normal appear to be inconclusive, because of several bias factors, which include differences in MCI neuropsychological criteria, transient medical comorbidities, or neuropsychiatric symptoms that might lead to false positive results, length of follow-up [53, 54].
NEUROPATHOLOGICAL BIOMARKERS OF ALZHEIMER’S DISEASE AND RELATED CONDITIONS
Neuropathological changes associated with AD begin years or even decades before AD symptoms and signs appear and can be documented in the pre-symptomatic AD stage [55]. According to this view, amnestic MCI coupled with positive biomarkers of amyloid or amyloid and tau neuropathology represents the earliest symptomatic stages of AD, since progressive neuronal loss leading to irreversible cognitive impairment may have already occurred [15, 55, 56]. There are, however, a number of open questions pertaining to the association between AD pathological changes and the onset of AD symptoms. A frailty accumulation index was reported to modulate the relationship between AD neuropathologic changes and dementia in a cohort of adults older than 59 years without known dementia at baseline, suggesting that individuals with even a low level of AD-related pathology might be at risk for dementia if they have high amounts of frailty [57].
The recent NIA-AA Research Framework introduced the A/T/N classification system, a biomarker classification scheme that groups different biomarkers (imaging and biofluids) by the pathologic process each measure [58, 59], and changed the view of AD pathology from a clinical-neuropathological to a clinical-biomarker entity. A/T/N classification divides AD biomarkers into three categories, according to the nature of the measured pathological process: A stands for amyloid biomarkers, T stands for tau biomarkers, and N stands for neurodegeneration or neuronal injury biomarkers [58]. Each type of biomarkers has different kind of representatives (Table 2) and is rated as positive or negative based on their presence in a single subject [59]. On the basis of the A/T/N classification scheme, the NIAA-AA Research Framework proposed AD staging across a continuum, and identified eight different biomarker profiles and three biomarker categories: individuals with normal AD biomarkers, individuals in the AD continuum (i.e., AD pathological change); non–AD pathological changes (i.e., with normal A biomarker but abnormal T and/or N; Table 3) [59]. The combination of cognitive status according to neuropsychological testing and the biomarker profiles according to the NIAA-AA Research Framework could lead to a better understanding of the complexity of AD and non-AD conditions, by focusing on the neuropathological features in parallel to the clinical/cognitive ones [59].
Table 2
Biomarker classes according to the A/T/N classification system [59]
| Biomarker class | Marker (CSF/imaging) |
| A (Amyloid) | CSF Aβ42 |
| Amyloid imaging | |
| T (Tau) | CSF phosphorylated tau |
| Tau imaging | |
| N (Neurodegeneration or neuronal injury) | CSF total tau |
| Magnetic resonance imaging | |
| 18-FDG positron emission tomography |
Aβ42, amyloid-β protein 42 peptide; CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose.
Table 3
Biomarker profiles and Alzheimer’s disease continuum [58, 59]
| Amyloid (A) | Tau (T) | Neurodegeneration or neuronal injury (N) | Category | NIA-AA Staging |
| Normal AD biomarkers | ||||
| – | – | – | Normal | Not defined |
| AD continuum | ||||
| + | – | – | AD pathological change | Stage 1 |
| + | + | – | AD | Stage 2/3 |
| + | + | + | AD | Stage 2/3 |
| + | – | + | AD and suspected concomitant non–AD pathological change | Not applicable |
| Non–AD pathological changes | ||||
| – | + | – | Non–AD pathological change | Not defined |
| – | – | + | Non–AD pathological change | Not defined |
| – | + | + | Non–AD pathological change | Not defined |
AA, Alzheimer’s Association; AD, Alzheimer’s disease; NIA, National Institute on Aging.
The NIA-AA Research Framework proposed a six-level clinical staging that corresponds to the classical clinical syndromes (i.e., cognitively unimpaired, SCD or transitional cognitive decline, MCI, mild-to-severe dementia) and should be applicable only to individuals in the AD continuum (Table 4) [59]. Clinical stages do not run in parallel with the neuropathological changes, because AD biomarkers may be positive independently on the severity of clinical staging, e.g., cognitively unimpaired or SCD patients may have positive amyloid biomarkers [59].
Table 4
Clinical staging and related clinical syndromes in the Alzheimer’s disease continuum [59]
| Stage | Clinical syndrome | Clinical criteria |
| 1 | CU | No reported decline in cognition or new onset of neurobehavioral symptoms |
| Cognitive performance within expected range on testing | ||
| 2 | SCD/TCD | Self-experienced persistent decline in cognition compared to baseline and/or mild behavioral changes*, † |
| No functional impact on daily life activities | ||
| Cognitive performance within expected range on testing | ||
| 3 | MCI | Self-experienced persistent decline in cognition compared to baseline and/or mild behavioral changes*, † |
| Preserved independence in the daily life activities and/or mild impact on the more complex ones | ||
| Cognitive performance below expected range on testing | ||
| 4 | Dementia | Progressive cognitive impairment affecting several cognitive domains (mild to severe) and/or neurobehavioral symptoms |
| 5 | Significant functional impact on activities of daily life | |
| 6 | Mild (stage 4), moderate (stage 5) or severe (stage 6) according to the degree of cognitive, behavioral and functional involvement |
Note that the clinical stages and syndromes do not run in parallel with the neuropathological changes. *The self-experienced persistent decline in cognitive functioning and/or behavioral changes must be unrelated to an acute event. †Reported by the patient or by an informant. CU, cognitively unimpaired; MCI, mild cognitive impairment; NIA, National Institute on Aging; SCD, subjective cognitive decline; TCD, transitional cognitive decline.
A greater number of abnormal AD biomarkers, indicating more severe AD pathology, have been reported to result in a higher risk of short-term cognitive decline [59]. We may hypothesize that a greater amount of AD biomarker changes at ages younger than 70 could characterize CF, although the longitudinal trajectories of AD biomarkers have not been investigated, to date [60]. Indeed, biomarker-based research should not be considered a template for all research into age-related cognitive impairment and dementia [59] and our hypothesis should be further explored in clinical trials.
Pre-frailty and frailty could represent a part of the same functional decline, and an early diagnosis and intervention could be possible considering the physical performances in addition to the cognitive framework of frailty.
COGNITIVE FRAILTY IN THE ERA OF ALZHEIMER’S DISEASE BIOMARKERS
The introduction of AD biomarkers changed the significance of MCI as a diagnostic category and the view that this condition may be reversible. According to the NIA-AA Research Framework [59], MCI patients with positive A biomarkers should be diagnosed as AD pathologic change with MCI, while those with positive A and T biomarkers should be diagnosed as prodromal AD, irrespective of N biomarkers status. Therefore, the term reversibility should be applied to MCI only if modifiable factors, such as polypharmacy, psychiatric conditions (e.g., depression), metabolic deficiencies, sleep disturbances, or sensory deficits could be identified [61]. These considerations imply that identifying MCI with CF and considering MCI as potentially reversible CF, as stipulated by the IANA-IAGG [36] and the Ruan et al. [33] definitions, without considering the underscoring neuropathology may be too simplistic and not reflect the multifaceted nature of this condition.
According to the definition by Ruan and collaborators [33], reversible CF corresponds to SCD, a non-specific condition characterized by self-report of persistent decline in cognitive capacity in comparison with a previously normal status and unrelated to an acute event, without objective impairment detected by standardized neuropsychological tests [41]. SCD may result from multiple causes, such as normal aging, preclinical AD, other psychiatric and neurologic disorders, side effect of drugs and substance use disorders (SUD) [41, 62, 63]. Indeed, neurobehavioral changes should have a clearly defined recent onset, be persistent and not explained by life events [59]. Despite the absence of objective evidence of cognitive impairment, reversibility of SCD might be questionable in the presence of AD biomarkers [64], particularly in younger patients. Patients with SCD and positive A with or without T biomarkers corresponds to preclinical AD pathological changes or preclinical AD [59] that have a high likelihood of converting to overt AD [56, 65–67]. However, since neurodegeneration and neuronal damage may be negative in SCD cases and functional compensation is still possible [41], this population might be the most suitable for interventions aimed at preventing or at least postponing progression to dementia (Fig. 1). Most individuals with SCD appear not to follow the temporal biomarker order proposed by NIA-AA criteria, and a more parsimonious staging approach that does not presume all patients follow a singular invariant expression of the disease has been proposed [68].
Fig.1
Cognitive frailty and the natural history of Alzheimer’s disease (AD). Here is schematically depicted the natural history of AD with the supposed timing of neuropathological changes in relation to the cognitive decline [59]. The definition of patients with subjective cognitive decline (SCD) and early mild cognitive impairment (MCI) at risk of dementia in the cognitive frailty stage could offer a potential therapeutic window to overcome the limitations of the current unsuccessful therapeutic window of late MCI and early dementia.
![Cognitive frailty and the natural history of Alzheimer’s disease (AD). Here is schematically depicted the natural history of AD with the supposed timing of neuropathological changes in relation to the cognitive decline [59]. The definition of patients with subjective cognitive decline (SCD) and early mild cognitive impairment (MCI) at risk of dementia in the cognitive frailty stage could offer a potential therapeutic window to overcome the limitations of the current unsuccessful therapeutic window of late MCI and early dementia.](https://content.iospress.com:443/media/jad/2020/76-3/jad-76-3-jad200137/jad-76-jad200137-g001.jpg)
Cognitive impairment or decline, however, is not result of AD pathological changes alone, because it is often the result of mixed pathology, including AD and other concurrent degenerative pathology and/or vascular pathology and the scenario appears to be more complex and the topic for future research.
OPERATIONAL CRITERIA FOR COGNITIVE FRAILTY
The absence of robust and widely accepted operational criteria may limit the spread of the concept of CF [69]. At variance with the physical components of CF that were clearly operationalized by the Cardiovascular Health Study, the cognitive features of this construct were not well described, in that they include comorbid dementia, signs or symptoms of cognitive dysfunction, clouding, or delirium [42, 70]. Tools to detect CF were reported in approximately one third of studies and vary from self-reported cognitive-screening questionnaires to screening tests and neuropsychological batteries [71, 72]. Mini-Mental State Examination (MMSE) is the most widely used screening test, despite it has been shown to fail in detecting mild cognitive disturbances [73]; similarly, CDR, although widely adopted, may not be adequate, because it is too simple and not sensitive to mild cognitive deficits. Moreover, since they were developed as screening tools for AD-related dementia, MMSE and CDR are mainly focused on memory, being poorly sensitive to other cognitive domains, in particular to executive functions. To overcome this drawback, some researchers proposed the use of a wide range of neuropsychological tests [74], such as the frontal assessment battery [75], the five words test [76], the trail making test [77], the free and cued selective reminding tests [78], the digit symbol substitution subtest of the Wechsler adult intelligence scale revised [79], and the verbal fluency test [80], but they explore different cognitive domains with variable sensibility, specificity, reliability, and validity and some of them may be sensitive to the age of the patient [71, 81]. Since patients with CF may show mild levels of cognitive impairment, a more in-depth neuropsychological assessment including at least one test for cognitive domain would be more sensitive and could help understand if different CF subtypes can be documented.
An operational definition of SCD that captures both cognitive and functional decline has been recently proposed [68]. The analysis of qualitative and strategic aspects of cognitive performance such as process scores analysis, word-list intrusion errors, retroactive interference, and learning slope in SCD patients may increase the sensitivity and the earlier identification of cognitively normal older adults at risk for decline [82]. These findings suggest that widely accepted operational criteria and an in-depth neuropsychological evaluation may improve the prognostic evaluation of SCD patients.
Indeed, these figures indicate the absence of gold standard operational criteria for detecting CF, thus making the comparison of findings across studies difficult [71, 83]. Referring to common operational criteria for CF would be important to offer psychometrically adequate and widely shared clinical measures.
THE ASSOCIATION BETWEEN COGNITIVE AND PHYSICAL FRAILTY
The possible common etiopathogenesis between cognitive and physical frailty is a key feature on this topic that still remains unresolved [84]. Current definitions of CF stipulate physical pre-frailty or frailty in association with cognitive impairment [33, 36]. CF has been proposed as a potentially reversible clinical entity and represents an important target of secondary intervention in early or asymptomatic stage of dementia to promote healthy aging [85]. Common underlying mechanisms between physical and cognitive impairment include neuropathological changes, cardiovascular elements, nutrition, hormones, and chronic inflammation [36, 86, 87]. A significant correlation between frailty and the global cortical atrophy was reported [88], and a CF model was demonstrated to have significant additional predictive effect on the risk of disability than the physical frailty model only [89]. Several cross-sectional studies demonstrated the interconnection between physical frailty and cognitive performance in people aged >65 years, but data about the possible causal or temporal relationship between these two components are scarce [70], and their dissimilar evolution suggest a more open view when considering their possible relationship [42]. Indeed, the prevalence of frailty according to the Fried’s criteria is on average less than 5% under the age of 70 years [90]. At variance, SCD and MCI frequently affect people younger than 70 years. A recent study reported the prevalence of SCD to be around 10% in adults aged 45–54 years with functional limitations in nearly 60% of those reporting SCD [91]. The different prevalence of physical frailty and SCD across the life span suggests that the concept of CF should be expanded to middle-aged adults with SCD, who could represent the most important population where therapeutic strategies to reduce the risk of conversion to dementia should be tested, as discussed above. This view might increase our understanding of how frailty and its risk factors develop during the earlier stages in life and contribute to the development of public health strategies aimed at preventing frailty and related adverse health outcomes [90]. Whether this approach is cost-effective should be the topic of future studies.
There is increasing evidence that poor gait performance is strongly associated with the incidence of cognitive decline and dementia, especially non-AD dementia that led to the definition of motoric cognitive risk syndrome, defined as presence of both slow gait and subjective cognitive complaints and absence of concurrent dementia or mobility disability [92]. A simple motor test of gait velocity combined with a reliable cognitive test was found to be superior than the CF construct (i.e., physical frailty plus CDR score = 0.5) to detect individuals at risk for dementia [93]. The correlation between lower limb motor function and the risk of dementia further casts doubt on the empirical basis of the CF syndrome [94]. These findings underscore that robust operational criteria for CF are needed, as discussed above. Longitudinal studies have indeed led to inconclusive results, underscoring both the parallelism and the dissonances between physical and CF, impeding firm conclusions on the correlation between the two components [42, 95, 96].
Whether physical frailty and cognitive impairment represent a unique phenotype, as suggested by the CF construct, should be considered different phenotypes of a common underlying mechanism (i.e., vascular diseases, neurodegeneration), or are two separate conditions, co-occurring in older age, are still open questions with no definitive answer according to current knowledge.
Moreover, since subjects with SCD are often younger, they may complain of cognitive symptoms in the absence of physical disturbances. However, studies addressing the association between physical and CF in people aged less than 65 years are still lacking, probably because younger patients may be more unlikely to fulfill the criteria for physical frailty. Therefore, physical and CF should probably be kept separated in subjects <65 years.
COGNITIVE FRAILTY: TOWARDS A MULTIDIMENSIONAL REDEFINITION
Cognitive decline due to neurodegenerative disorders and coexisting vascular changes is expected to become one of the major health issues in the next few years [7, 97]. Because of the limited efficacy of currently available treatments for AD, the identification of potentially modifiable factors to be targeted by preventive and interventional strategies is gaining increasing attention. Among them, CF and associated physical frailty seem to represent a potential promising candidate. The current CF definitions present some conceptual and methodological issues that have been discussed above. Here we offer some proposals for updating and improving the CF construct.
First, the view that CF could be reversible or potentially reversible, either if the cognitive profile of the patient is MCI or SCD, should not rely upon clinical and neuropsychological profile only, but should take into account the presence or absence of AD biomarkers, according to the A/T/N classification system [58, 59] and other neurodegenerative or vascular changes, especially in younger patients, where these changes have a more robust pathological significance. At variance with Ruan’s definition of CF [33], some caution should be paid in considering SCD or MCI patients with positive biomarkers as reversible CF. In such cases, potentially modifiable factors, such as psychiatric comorbidities (e.g., depression or dysthymia), psychosocial (e.g., social isolation), biological (e.g., changes related to aging, metabolic deficits), or pharmacological factors (e.g., side effect of drugs, SUD) could represent the target for interventions aimed to reverse CF [41, 98]. In any case, the clinical construct of CF should be kept separated from the neuropathological changes and biomarkers, which should include the A/T/N framework, the other neurodegenerative and vascular changes. Indeed, the proposed CF construct differs from the SCD/MCI ones, in that operational criteria are available for the latter, but needs to be defined for the former. What’s more, CF may coexist with physical frailty, especially in elderly people.
Second, because of the absence of clear evidence that physical and cognitive components of frailty are correlated, they should better be considered as separate entities, instead of part of a single macro-phenotype [33].
Third, despite studies on physical frailty traditionally focused on patients older than 65 years, CF should probably not be considered a condition limited to geriatric population. Trajectories of amyloid accumulation are supposed to start earlier than 65 years and identifying patients with CF at higher risk of conversion to dementia may be important for future disease-modifying treatments [18]. It is worthy of note that CF does not simply reflect a condition of increased brain vulnerability due to aging. Brain aging indeed results in an unbalance between functional network integration and segregation in the medial temporal lobe memory and the frontostriatal executive systems, representing a physiological process without necessarily an overt cognitive impairment [99]. Conversely, CF refers to brain frailty that may be associated to neuropathological changes related to AD, cerebrovascular diseases, or other neurodegenerative conditions, making people more susceptible to cognitive, as well as motor, decline. Moreover, CF may affect people younger than 65 years.
Based on the lines of reasoning reported above, a proposal for a revised definition of CF according to a multidimensional subtyping is presented in Table 5 and Fig. 2.
Table 5
Proposal for a revised definition of cognitive frailty according to a multidimensional model
| Clinical CF construct | Neuropathology and biomarkers | |||
| CF types | CF subtypes/dimensions | |||
| Age group | Physical frailty | Coexisting conditions† | ||
| SCD-CF | <65 years (older adults) | Physical frailty+/– | Psychiatric disorders+/– | A/T/N* |
| 65–80 years (elderly people) | Side effects of drugs or SUD+/– | Other neurodegeneration | ||
| >80 years (oldest-old people) | Other conditions+/– | Vascular | ||
| MCI-CF | <65 years (older adults) | Physical frailty+/– | Psychiatric disorders+/– | A/T/N* |
| 65–80 years (elderly people) | Side effects of drugs or SUD+/– | Other neurodegeneration | ||
| >80 years (oldest-old people) | Other conditions+/– | Vascular | ||
A, amyloid; CF, cognitive frailty; MCI, mild cognitive impairment; N, neurodegeneration or neuronal injury; SCD, subjective cognitive decline; SUD, substance use disorders; T, tau. †Neurobehavioral changes should have a clearly defined recent onset, be persistent and not explained by life events [59]. *Not all A/T/N conditions could be considered as CF, since A+/T+ and A+/N+ conditions should more appropriately fit into Alzheimer’s disease continuum [59]; caution should be taken for the oldest-old people because these changes might be present despite normal cognition.
Fig.2
The proposed multidimensional clinical construct of cognitive frailty and the parallel neuropathological changes and biomarkers. Clinical construct of cognitive frailty should include subjective cognitive decline (SCD) or mild cognitive impairment (MCI) together with physical frailty and should consider age range and comorbidities (i.e., psychiatric, drug-related and other coexisting conditions). The neuropathological changes and biomarkers, if present, may offer additional prognostic information, e.g., stratifying the risk of conversion to dementia.
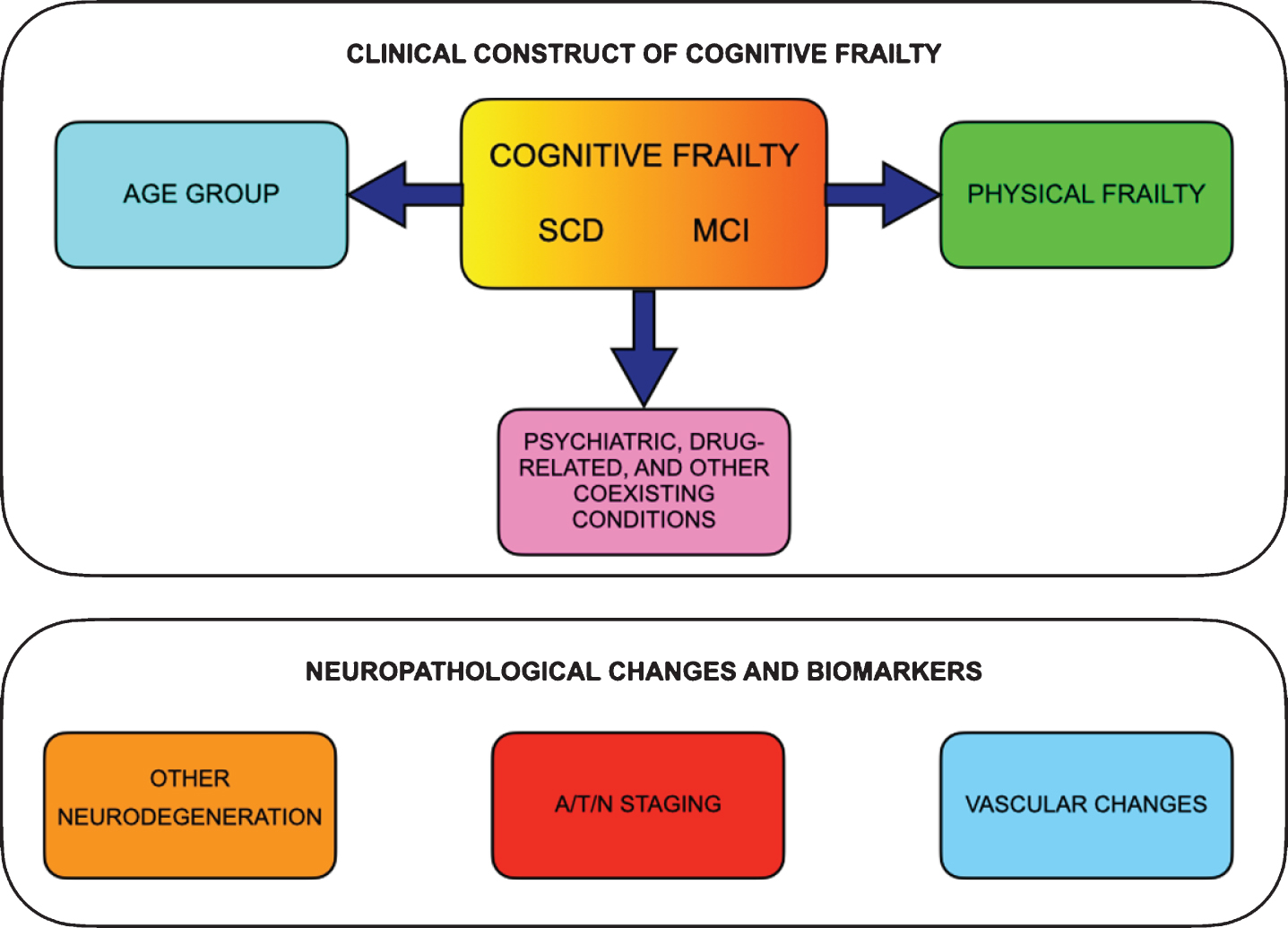
Finally, operational criteria for CF are important to offer a standardized assessment. In analogy to physical frailty [100], a simple, valid, reliable, and sensitive questionnaire, checklist, or abbreviated screening tool could identify patients that should undergo further testing throughout a comprehensive neuropsychological examination. In the absence of a gold standard neuropsychological measure of CF, future directions for clinical research should deal with the identification of the best neuropsychological battery with high sensitivity and specificity and short time for administration that could stratify CF patients according to the most involved cognitive domains and be tailored to patient’s age.
These changes to CF definition might help stratifying CF patients with a broader perspective to design future trials aimed at exploring lifestyle interventions for risk factors and mechanisms involved in conversion to dementia [101] and to test disease-modifying pharmacological strategies to target AD neuropathological changes at an earlier disease stages than they are currently performed [18]. Future studies should explore if the proposed redefinition of CF offers advantages in comparison to the simpler constructs of MCI and SCD, and if it can be easily applied in the clinical setting in diagnostic and therapeutic studies.
ACKNOWLEDGMENTS
The present study has been partially supported by the Italian Ministry of Research and University (MIUR) 5-year special funding to strengthen and enhance the excellence in research and teaching (https://www.miur.gov.it/dipartimenti-di-eccellenza). The funding source had no role in study design, in the collection, analysis, and interpretation of data, in writing of the report, and in the decision to submit the article for publication.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0137r2).
REFERENCES
[1] | Zucchella C , Consilvio M , Iacoviello L , Intiso D , Tamburin S , Casale R , Bartolo M ((2019) ) Rehabilitation in oldest-old stroke patients: A comparison within over 65 population. Eur J Phys Rehabil Med 55: , 148–155. |
[2] | Lopez-Otin C , Blasco MA , Partridge L , Serrano M , Kroemer G ((2013) ) The hallmarks of aging. Cell 153: , 1194–1217. |
[3] | Flatt T , Partridge L ((2018) ) Horizons in the evolution of aging. BMC Biol 16: , 93. |
[4] | Moskalev AA , Aliper AM , Smit-McBride Z , Buzdin A , Zhavoronkov A ((2014) ) Genetics and epigenetics of aging and longevity. Cell Cycle 13: , 1063–1077. |
[5] | Clegg A , Young J , Iliffe S , Rikkert MO , Rockwood K ((2013) ) Frailty in elderly people. Lancet 381: , 752–762. |
[6] | Niccoli T , Partridge L ((2012) ) Ageing as a risk factor for disease. Curr Biol 22: , R741–R52. |
[7] | Prince M , Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer Disease International, London, UK. |
[8] | Salomon JA , Wang H , Freeman MK , Vos T , Flaxman AD , Lopez AD , Murray CJ ((2012) ) Healthy life expectancy for 187 countries, 1990–2010: A systematic analysis for the Global Burden Disease Study 2010. Lancet 380: , 2144–2162. |
[9] | Bousquet J , Kuh D , Bewick M , Standberg T , Farrell J , Pengelly R , Joel ME , Rodriguez Mañas L , Mercier J , Bringer J , Camuzat T , Bourret R , Bedbrook A , Kowalski ML , Samolinski B , Bonini S , Brayne C , Michel JP , Venne J , Viriot-Durandal P , Alonso J , Avignon A , Ben-Shlomo Y , Bousquet PJ , Combe B , Cooper R , Hardy R , Iaccarino G , Keil T , Kesse-Guyot E , Momas I , Ritchie K , Robine JM , Thijs C , Tischer C , Vellas B , Zaidi A , Alonso F , Andersen Ranberg K , Andreeva V , Ankri J , Arnavielhe S , Arshad H , Augé P , Berr C , Bertone P , Blain H , Blasimme A , Buijs GJ , Caimmi D , Carriazo A , Cesario A , Coletta J , Cosco T , Criton M , Cuisinier F , Demoly P , Fernandez-Nocelo S , Fougère B , Garcia-Aymerich J , Goldberg M , Guldemond N , Gutter Z , Harman D , Hendry A , Heve D , Illario M , Jeandel C , Krauss-Etschmann S , Krys O , Kula D , Laune D , Lehmann S , Maier D , Malva J , Matignon P , Melen E , Mercier G , Moda G , Nizinkska A , Nogues M , O’Neill M , Pelissier JY , Poethig D , Porta D , Postma D , Puisieux F , Richards M , Robalo-Cordeiro C , Romano V , Roubille F , Schulz H , Scott A , Senesse P , Slagter S , Smit HA , Somekh D , Stafford M , Suanzes J , Todo-Bom A , Touchon J , Traver-Salcedo V , Van Beurden M , Varraso R , Vergara I , Villalba-Mora E , Wilson N , Wouters E , Zins M ((2015) ) Operational definition of Active and Healthy Ageing (AHA): A conceptual framework. J Nutr Health Aging 19: , 955–960. |
[10] | Martin P , Kelly N , Kahana B , Kahana E , Willcox BJ , Willcox DC , Poon LW ((2015) ) Defining successful aging: A tangible or elusive concept? Gerontologist 55: , 14–25. |
[11] | ZucchellaC, SinforianiE, TamburinS, FedericoA, MantovaniE, BerniniS, CasaleR, BartoloM ((2018) ) The multidisciplinary approach to Alzheimer’s disease and dementia. a narrative review of non-pharmacological treatment. Front Neurol 9: , 1058. |
[12] | Scheltens P , Blennow K , Breteler MM , de Strooper B , Frisoni GB , Salloway S , Van der Flier WM ((2016) ) Alzheimer’s disease. Lancet 388: , 505–517. |
[13] | Coleman PD , Mastroeni D ((2017) ) A call for new approaches to Alzheimer’s disease research. Neurobiol Aging 57: , iii–iv. |
[14] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[15] | Sperling RA , Jack CR Jr , Aisen PS ((2011) ) Testing the right target and right drug at the right stage. Sci Transl Med 3: , 111cm33. |
[16] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[17] | Madav Y , Wairkar S , Prabhakar B ((2019) ) Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res Bull 146: , 171–184. |
[18] | Andrieu S , Coley N , Lovestone S , Aisen PS , Vellas B ((2015) ) Prevention of sporadic Alzheimer’s disease: Lessons learned from clinical trials and future directions. Lancet Neurol 14: , 926–944. |
[19] | Reiman EM , Langbaum JB , Tariot PN , Lopera F , Bateman RJ , Morris JC , Sperling RA , Aisen PS , Roses AD , Welsh-Bohmer KA , Carrillo MC , Weninger S ((2016) ) CAP–advancing the evaluation of preclinical Alzheimer disease treatments. Nat Rev Neurol 12: , 56–61. |
[20] | Reiman EM , Langbaum JB ((2009) ) Brain imaging in the evaluation of putative Alzheimer’s disease slowing, risk-reducing and prevention therapies. In Imaging the Aging Brain, JagustWJ, D’EspositoM, eds. Oxford University Press, pp. 319–350. |
[21] | Bateman RJ , Aisen PS , De Strooper B , Fox NC , Lemere CA , Ringman JM , Salloway S , Sperling RA , Windisch M , Xiong C ((2011) ) Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther 3: , 1. |
[22] | Emery VO ((2011) ) Alzheimer disease: Are we intervening too late? J Neural Transm (Vienna) 118: , 1361–1378. |
[23] | Sugimoto T , Sakurai T , Ono R , Kimura A , Saji N , Niida S , Toba K , Chen LK , Arai H ((2018) ) Epidemiological and clinical significance of cognitive frailty: A mini review. Ageing Res Rev 44: , 1–7. |
[24] | Gobbens RJ , Luijkx KG , Wijnen-Sponselee MT , Schols JM ((2010) ) In search of an integral conceptual definition of frailty: Opinions of experts. J Am Med Dir Assoc 11: , 338–343. |
[25] | Fitten JL ((2015) ) Thinking about cognitive frailty. J Prev Alz Dis 2: , 7–10. |
[26] | Roppolo M , Mulasso A , Gobbens RJ , Mosso CO , Rabaglietti E ((2015) ) A comparison between uni- and multidimensional frailty measures: Prevalence, functional status, and relationships with disability. Clin Interv Aging 10: , 1669–1678. |
[27] | Cesari M , Calvani R , Marzetti E ((2017) ) Frailty in older persons. Clin Geriatr Med 33: , 293–303. |
[28] | Tabue-Teguo M , Simo N , Gonzalez-Colaço Harmand M , Cesari M , Avila-Funes JA , Féart C , Amiéva H , Dartigues JF ((2017) ) Frailty in elderly: A brief review. Geriatr Psychol Neuropsychiatr Vieil 15: , 127–137. |
[29] | Fried LP , Tangen CM , Walston J , Newman A , Hirsch C , Gottdiener J , Seeman T , Tracy R , Kop WJ , Burke G , McBurnie MA ((2001) ) Frailty in older adults: Evidence for a phenotype. J Gerontol Series A Biol Sci Med Sci 56: , 146–156. |
[30] | Rockwood K , Song X , MacKnight C , Bergman H , Hogan DB , McDowell I , Mitnitski A ((2005) ) A global clinical measure of fitness and frailty in elderly people. CMAJ 173: , 489–495. |
[31] | Gobbens RJ , Luijkx KG , Wijnen-Sponselee MT , Schols JM ((2010) ) Towards an integral conceptual model of frailty. J Nutr Health Aging 14: , 175–181. |
[32] | Solfrizzi V , Scafato E , Lozupone M , Seripa D , Schilardi A , Custodero C , Sardone R , Galluzzo L , Gandin C , Baldereschi M , Di Carlo A , Inzitari D , Giannelli G , Daniele A , Sabbà C , Logroscino G , Panza F ; Italian Longitudinal Study on Aging Working Group ((2019) ) Biopsychosocial frailty and the risk of incident dementia: The Italian longitudinal study on aging. Alzheimers Dement 15: , 1019–1028. |
[33] | Ruan Q , Yu Z , Chen M , Bao Z , Li J , He W ((2015) ) Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res 20: , 1–10. |
[34] | Bunt S , Steverink N , Olthof J , van der Schans CP , Hobbelen JS ((2017) ) Social frailty in older adults: A scoping review. Eur J Ageing 14: , 323–334. |
[35] | Panza F , D’Introno A , Colacicco AM , Capurso C , Parigi AD , Capurso SA , Caselli RJ , Pilotto A , Scafato E , Capurso A , Solfrizzi V ((2006) ) Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiol Aging 27: , 933–940. |
[36] | Kelaiditi E , Cesari M , Canevelli M , van Kan GA , Ousset PJ , Gillette-Guyonnet S , Ritz P , Duveau F , Soto ME , Provencher V , Nourhashemi F , Salva A , Robert P , Andrieu S , Rolland Y , Touchon J , Fitten JL , Vellas B ((2013) ) Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging 17: , 726–734. |
[37] | Moeley JE , Vellas B , van kan GA , Anker SD , Bauer JM , Bernabei R , Cesari M , Chumlea WC , Doehner W , Evans J , Fried LP , Guralnik JM , Katz PR , Malmstrom TK , McCarter RJ , Gutierrez Robledo LM , Rockwood K , von Haehling S , Vandewoude MF , Walston J ((2013) ) Frailty consensus: A call to action. J Am Med Dir Assoc 14: , 392–397. |
[38] | Clegg A , Barber S , Young J , Iliffe S , Forster A ((2014) ) The home-based older people’s exercise (HOPE) trial: A pilot randomised controlled trial of a home-based exercise intervention for older people with frailty. Age Ageing 43: , 687–695. |
[39] | Dorner TE , Lackinger C , Haider S , Luger E , Kapan A , Luger M , Schindler KE ((2013) ) Nutritional intervention and physical training in malnourished frail community-dwelling elderly persons carried out by trained lay buddies: Study protocol of a randomized controlled trial. BMC Public Health 27: , 1232. |
[40] | Tavassoli N , Guyonnet S , Abellan Van Kan G , Sourdet S , Krams T , Soto ME , Subra J , Chicoulaa B , Ghisolfi A , Balardy L , Cestac P , Rolland Y , Andrieu S , Nourhashemi F , Oustric S , Cesari M , Vellas B , Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability Team ((2014) ) Description of 1,108 older patients referred by their physician to the Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability at the gerontopole. J Nutr Health Aging 18: , 457–464. |
[41] | Jessen F , Amariglio RE , van Boxtel M , Breteler M , Ceccaldi M , Chetelat G , Dubois B , Dufouil C , Ellis KA , van der Flier WM , Glodzik L , van Harten AC , de Leon MJ , McHugh P , Mielke MM , Molinuevo JL , Mosconi L , Osorio RS , Perrotin A , Petersen RC , Rabin LA , Rami L , Reisberg B , Rentz DM , Sachdev PS , de la Sayette V , Saykin AJ , Scheltens P , Shulman MB , Slavin MJ , Sperling RA , Stewart R , Uspenskaya O , Vellas B , Visser PJ , Wagner M ; Subjective Cognitive Decline Initiative (SCD-I) Working Group ((2014) ) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10: , 844–852. |
[42] | Facal D , Maseda A , Pereiro AX , Gandoy-Crego M , Lorenzo-López L , Yanguas J , Millán-Calenti JC ((2019) ) Cognitive frailty: A conceptual systematic review and an operational proposal for future research. Maturitas 121: , 48–56. |
[43] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[44] | Kapasi A , DeCarli C , Schneider JA ((2017) ) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134: , 171–186. |
[45] | Petersen RC , Bennett D ((2005) ) Mild cognitive impairment: Is it Alzheimer’s disease or not? J Alzheimers Dis 7: , 241–262. |
[46] | Borroni B , Cosseddu M , Pilotto A , Premi E , Archetti S , Gasparotti R , Cappa S , Padovani A ((2015) ) Early stage of behavioral variant frontotemporal dementia: Clinical and neuroimaging correlates. Neurobiol Aging 36: , 3108–3115. |
[47] | Sadiq D , Whitfield T , Lee L , Stevens T , Costafreda S , Walker Z ((2017) ) Prodromal dementia with Lewy Bodies and prodromal Alzheimer’s disease: A comparison of the cognitive and clinical profiles. J Alzheimers Dis 58: , 463–470. |
[48] | MitchellAJ, Shiri-FeshkiA ((2009) ) Rate of progression of mild cognitive impairment to dementia—meta- analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119: , 252–265. |
[49] | Roberts RO , Knopman DS , Mielke MM , Cha RH , Pankratz VS , Christianson TJ , Geda YE , Boeve BF , Ivnik RJ , Tangalos EG , Rocca WA , Petersen RC ((2014) ) Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 82: , 317–325. |
[50] | Alves L , Cardoso S , Maroco J , de Mendonca A , Guerreiro M , Silva D ((2018) ) Neuropsychological predictors of long-term (10 years) mild cognitive impairment stability. J Alzheimers Dis 62: , 1703–1711. |
[51] | Manly JJ , Tang MX , Schupf N , Stern Y , Vonsattel JP , Mayeux R ((2008) ) Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 63: , 494–506. |
[52] | Han JW , Kim TH , Lee SB , Park JH , Lee JJ , Huh Y , Park JE , Jhoo JH , Lee DY , Kim KW ((2012) ) Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement 8: , 553–559. |
[53] | Petersen RC , Caracciolo B , Brayne C , Gauthier S , Jelic V , Fratiglioni L ((2014) ) Mild cognitive impairment: A concept in evolution. J Intern Med 275: , 214–228. |
[54] | Pandya SY , Lacritz LH , Weiner MF , Deschner M , Woon FL ((2017) ) Predictors of reversion from mild cognitive impairment to normal cognition. Dement Geriatr Cogn Disord 43: , 204–214. |
[55] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[56] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[57] | Wallace LMK , Theou O , Godin J , Andrew MK , Bennett DA , Rockwood K ((2019) ) Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: A cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol 18: , 177–184. |
[58] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Feldman HH , Frisoni GB , Hampel H , Jagust WJ , Johnson KA , Knopman DS , Petersen RC , Scheltens P , Sperling RA , Dubois B ((2016) ) A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87: , 539–547. |
[59] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R ((2018) ) NIA-AA research framework: Towards a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[60] | Jack CR Jr , Wiste HJ , Weigand SD , Therneau TM , Knopman DS , Lowe V , Vemuri P , Mielke MM , Roberts RO , Machulda MM , Senjem ML , Gunter JL , Rocca WA , Petersen RC ((2017) ) Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: A cross-sectional study. Lancet Neurol 16: , 435–444. |
[61] | Sanford AM ((2017) ) Mild cognitive impairment. Clin Geriatr Med 33: , 325–337. |
[62] | Molinuevo JL , Rabin LA , Amariglio R , Buckley R , Dubois B , Ellis KA , Ewers M , Hampel H , Klöppel S , Rami L , Reisberg B , Saykin AJ , Sikkes S , Smart CM , Snitz BE , Sperling R , van der Flier WM , Wagner M , Jessen F ; Subjective Cognitive Decline Initiative (SCD-I) Working Group ((2017) ) Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement 13: , 296–311. |
[63] | Federico A , Tamburin S , Maier A , Faccini M , Casari R , Morbioli L , Lugoboni F ((2017) ) Multifocal cognitive dysfunction in high-dose benzodiazepine users: A cross-sectional study. Neurol Sci 38: , 137–142. |
[64] | Eliassen CF , Reinvang I , Selnes P , Grambaite R , Fladby T , Hessen E ((2017) ) Biomarkers in subtypes of mild cognitive impairment and subjective cognitive decline. Brain Behav 7: , e00776. |
[65] | van Oijen M , de Jong FJ , Hofman A , Koudstaal PJ , Breteler MM ((2007) ) Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimers Dement 3: , 92–97. |
[66] | Jessen F , Wiese B , Bachmann C , Eifflaender-Gorfer S , Haller F , Kölsch H , Luck T , Mösch E , van den Bussche H , Wagner M , Wollny A , Zimmermann T , Pentzek M , Riedel-Heller SG , Romberg HP , Weyerer S , Kaduszkiewicz H , Maier W , Bickel H ; German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group ((2010) ) Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 67: , 414–22. |
[67] | Reisberg B , Shulman MB , Torossian C , Leng L , Zhu W ((2010) ) Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 6: , 11–24. |
[68] | Edmonds EC , Delano-Wood L , Galasko DR , Salmon DP , Bondi MW ; Alzheimer’s Disease Neuroimaging Initiative ((2015) ) Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. J Alzheimers Dis 47: , 231–242. |
[69] | Ruan Q , D’Onofrio G , Sancarlo D , Greco A , Lozupone M , Seripa D , Panza F , Yu Z ((2017) ) Emerging biomarkers and screening for cognitive frailty. Aging Clin Exp Res 29: , 1075–1086. |
[70] | Roppolo M , Mulasso A , Rabaglietti E ((2017) ) Cognitive frailty in Italian community-dwelling older adults: Prevalence rate and its association with disability. J Nutr Health Aging 21: , 631–636. |
[71] | Vella Azzopardi R , Beyer I , Vermeiren S , Petrovic M , Van Den Noortgate N , Bautmans I , Gorus E , on behalf of the Gerontopole Brussels Study group ((2018) ) Increasing use of cognitive measures in the operational definition of frailty—A systematic review. Ageing Res Rev 43: , 10–16. |
[72] | Ruan Q , Xiao F , Gong K , Zhang W , Zhang M , Ruan J , Zhang X , Chen Q , Yu Z ((2020) ) Prevalence of cognitive frailty phenotypes and associated factors in a community-dwelling elderly population. J Nutr Health Aging 24: , 172–180. |
[73] | MitchellAJ ((2009) ) A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 43: , 411–431. |
[74] | Delrieu J , Andrieu S , Pahor M , Cantet C , Cesari M , Ousset P , Voisin T , Fougère B , Gillette S , Carrie I , Vellas B ((2016) ) Neuropsychological profile of cognitive frailty subjects in MAPT study. J Prev Alzheimers Dis 3: , 151–159. |
[75] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB: A frontal assessment battery at bedside. Neurology 55: , 1621–1626. |
[76] | Dubois B , Touchon J , Portet F , Ousset PJ , Vellas B , Michel B ((2002) ) The 5 words: A simple and sensitive test for the diagnosis of Alzheimer’s disease. Presse Med 31: , 1696–1699. |
[77] | Reitan R ((1958) ) Validity of the Trail Making Test as an indicator of brain damage. Percept Mot Skills 8: , 271–276. |
[78] | Grober E , Buschke H , Crystal H , Bang S , Dresner R ((1988) ) Screening for dementia by memory testing. Neurology 38: , 900–903. |
[79] | Wechsler D ((1981) ) Wechsler Adult Intelligence Scale-Revised, Psychological, New York. |
[80] | Cardebat D , Doyon B , Puel M , Goulet P , Joanette Y ((1990) ) Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg 90: , 207–217. |
[81] | ZucchellaC, FedericoA, MartiniA, TinazziM, BartoloM, TamburinS ((2018) ) Neuropsychological testing. Pract Neurol 18: , 227–237. |
[82] | Thomas KR , Edmonds EC , Eppig J , Salmon DP , Bondi MW ; Alzheimer’s Disease Neuroimaging Initiative ((2018) ) Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. J Alzheimers Dis 64: , 195–204. |
[83] | Sargent L , Brown R ((2017) ) Assessing the current state of cognitive frailty: Measurement properties. J Nutr Health Aging 21: , 152–160. |
[84] | Canevelli M , Cesari M ((2017) ) Cognitive frailty: Far from clinical and research adoption. J Am Med Dir Assoc 18: , 816–818. |
[85] | Panza F , Lozupone M , Solfrizzi V , Stallone R , Bellomo A , Greco A , Daniele A , Seripa D , Logroscino G ((2017) ) Cognitive frailty: A potential target for secondary prevention of dementia. Expert Opin Drug Metab Toxicol 13: , 1023–1027. |
[86] | Houles M , Canevelli M , van Kan GA , Ousset PJ , Cesari M , Vellas B ((2005) ) Frailty and cognition. J Frailty Aging 1: , 56–63. |
[87] | Robertson DA , Savva GM , Kenny RA ((2013) ) Frailty and cognitive impairment - a review of the evidence and causal mechanisms. Ageing Res Rev 12: , 840–851. |
[88] | Del Brutto OH , Mera RM , Cagino K , Fanning KD , Milla-Martinez MF , Nieves JL , Zambrano M , Sedler MJ ((2017) ) Neuroimaging signatures of frailty: A population-based study in community-dwelling older adults (the Atahualpa Project). Geriatr Gerontol Int 17: , 270–276. |
[89] | Solfrizzi V , Scafato E , Lozupone M , Seripa D , Giannini M , Sardone R , Bonfiglio C , Abbrescia DI , Galluzzo L , Gandin C , Baldereschi M , Di Carlo A , Inzitari D , Daniele A , Sabbà C , Logroscino G , Panza F ; Italian Longitudinal Study on Aging Working Group ((2017) ) Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: The Italian Longitudinal Study on Aging. Am J Geriatr Psychiatry 25: , 1236–1248. |
[90] | Hoogendijk EO , Afilalo J , Ensrud KE , Kowal P , Onder G , Fried LP ((2019) ) Frailty: Implications for clinical practice and public health. Lancet 394: , 1365–1375. |
[91] | Taylor CA , Bouldin ED , McGuire LC ((2018) ) Subjective cognitive decline among adults aged ≥45 years — United States, 2015–2016. MMWR Morb Mortal Wkly Rep 67: , 753–757. |
[92] | Verghese J , Annweiler C , Ayers E , Barzilai N , Beauchet O , Bennett DA , Bridenbaugh SA , Buchman AS , Callisaya ML , Camicioli R , Capistrant B , Chatterji S , De Cock AM , Ferrucci L , Giladi N , Guralnik JM , Hausdorff JM , Holtzer R , Kim KW , Kowal P , Kressig RW , Lim JY , Lord S , Meguro K , Montero-Odasso M , Muir-Hunter SW , Noone ML , Rochester L , Srikanth V , Wang C ((2014) ) Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology 83: , 718–726. |
[93] | Montero-Odasso MM , Barnes B Speechley M , Muir Hunter SW , Doherty TJ , Duque G , Gopaul K , Sposato LA , Casas-Herrero A , Borrie MJ , Camicioli R , Wells JL ((2016) ) Disentangling cognitive-frailty: Results from the Gait and Brain Study. J Gerontol A Biol Sci Med Sci 71: , 1476–1482. |
[94] | Kueper JK , Speechley M , Lingum NR , Montero-Odasso M ((2017) ) Motor function and incident dementia: A systematic review and meta-analysis. Age Ageing 46: , 729–738. |
[95] | Banks J , Batty GJ , Nazroo J , Steptoe A ((2016) ) The Dynamics of Ageing: Evidence from the English Longitudinal Study of Ageing 2002–2015. The Institute for Fiscal Studies, London. |
[96] | Feng L , Nyunt MS , Gao Q , Feng L , Lee TS , Tsoi T , Chong MS , Lim WS , Collinson S , Yap P , Yap KB , Ng TP ((2017) ) Physical frailty, cognitive impairment, and the risk of neurocognitive disorder in the Singapore Longitudinal Ageing Studies. J Gerontol A Biol Sci Med Sci 72: , 369–375. |
[97] | Brodaty H , Breteler MM , Dekosky ST , Dorenlot P , Fratiglioni L , Hock C , Kenigsberg PA , Scheltens P , De Strooper B ((2011) ) The world of dementia beyond 2020. J Am Geriatr Soc 59: , 923–927. |
[98] | Panza F , Lozupone M , Solfrizzi V , Sardone R , Dibello V , Di Lena L , D’Urso F , Stallone R , Petruzzi M , Giannelli G , Quaranta N , Bellomo A , Greco A , Daniele A , Seripa D , Logroscino G2 ((2018) ) Different cognitive frailty models and health- and cognitive-related outcomes in older age: From epidemiology to prevention. J Alzheimers Dis 62: , 993–1012. |
[99] | Jagust W ((2013) ) Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron 77: , 219–234. |
[100] | Morley JE , Malmstrom TK , Miller DK ((2012) ) A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 16: , 601–608. |
[101] | Kivipelto M , Mangialasche F , Ngandu T ((2018) ) Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14: , 653–666. |



