Abstract
Methanolic extract of Artemisia pallens (MEAP) (Asteraceae) was explored as greenbiocorrosion inhibitor for mild steel 1010 in 1.5% sodium chloride environment. Bacillus megaterium SKR7 induces the development of biofilm on the metal surface and forms the pitting corrosion. MEAP was showed (25 ppm) optimum inhibition effect of biocorrosion and further corrosion rate was highly reduced (0.3335 mm/year) than the control system (0.009 mm/year). The electrochemical study has supported the results with a higher value of total resistance (34 Ω cm2) when compared to control systems. It reveals the formation of a protective layer on the metal surface and reduces the adsorption of biofilm. This was due to the antimicrobial effect of MEAP. Overall, the results recognized that MEAP used as a green corrosion inhibitor for MS 1010 with 83% inhibition efficiency.
Similar content being viewed by others
Introduction
Currently, corrosion is one of the most dangerous issues in the metal industry worldwide. It is a destructive reaction between metal and oxygen with the results of electrochemical reactions in the metal oxide (Rajasekar et al. 2006; Narenkumar et al. 2019a). Various forms of corrosion are presented they are general (uniform), localized (pitting, crevice or under-deposit, intergranular, stress-cracking, galvanic), erosion (impingement and cavitation), dealloying, fatigue, filiform, fretting and microbially influenced corrosion (MIC) (Shi 2010; Chirkunov and Kuznetsov 2015; Matt Schnepf 2018). A cooling tower is the most important core element in the industrial process because it can aid in the ejection of heat absorb and removal of excess heat to the atmosphere by reducing the hot water to low temperature and circulating the water (Soares et al.2017). The principle of a cooling tower is water vaporization by the process of water is circulated on the top of the cooling tower (Tewari et al. 2019). The wastewater has used in the cooling water system to reclaimed the water again that is used for further industrial purposes. The wastewaters have a lot of nutrient content these are the basement of biofilm formation and microbes can use the nutrients for their attachment and growth (Aruliah and Ting 2014; Xu et al. 2012). The biofilm layer leads to corrosion and damages the cooling tower which resulting in a decrease in the flow rate, sample contamination, mechanism cross-reaction, finally system failure (George et al. 2016; Tewari et al. 2019). Due to the pH, temperature, nutrients, oxidation, and reduction are the main reason for microbial growth in the cooling tower (Telegdi et al. 2020). Most of the severe corrosion develops in a cooling system at 35 °C in that temperature microbes can replicate more copies (Xu et al. 2012). So, large numbers of microbes can accumulate the metal surface, equivalence charges are imbalanced that way exchange the ions of anode and cathode from the metals and occurred pitting corrosion ( Rodriguez-Leiva and Tributsch 1988; Telegdi et al. 2020). Microbes have two ways to penetrating the cooling tower, by the inflow of water or air that travels into the cooling tower. Bacteria, fungi, and algae are the microbes to cause the corrosion process in the cooling tower system (Tewari et al. 2019).
The MIC is mostly caused by pitting corrosion consequently microbes can accumulate specific place again and again (Atalah et al. 2020; Shi 2010). MIC is not a new form of corrosion, but to date, it is a more serious and dangerous problem of the industry. In 1891, the scientist Garrett studied microbial corrosion, it is the first report about microbes for corrosion. Microorganisms degrade metals due to oxidation and reduction of electrochemical reactions as called microbial influenced/induced corrosion. In 1943, scientist Zobell has proven appearance of the biofilm layer on the corrosive metal surface area and it has been a major reason for metal rusting, then the further innovative researches are started with this specific type of corrosion. Later, the researchers analysed the mechanism and investigated which types of microbes can be stimulated the microbially influenced corrosion (NACE 2005). Ford et al. (1990) reported major variations are available in binding ability among specific metal ions and unique exopolymers. The initial step of microbially influenced corrosion is the formation of biofilm (polymers) with their adhesion on the surface of metal ions (Moura et al. 2018; Zadvorny et al. 2006). Biofilm formation is a natural process of biofouling enhancement (Abdallah et al. 2014). Corrosion is occurred owing to the stimulation of microbe’s activities in the development of a gel matrix on the metals, particularly metabolism like EPS (extracellular polysaccharide substances) it includes proteins, polysaccharides, and nucleic acids of organisms. The microorganism behavior in the biofilm is triggering the metal's electrochemical open circuit potential (OCP) which can enhance the corrosion and it described such as Tafel polarization (TP), electrochemical impedance spectroscopy (EIS) (Tewari et al. 2019). Yearly 300–500 billion US dollars are economically loss of corrosion and 40% of internal corrosion caused by microorganisms both aerobic and anaerobic (Parthipan et al. 2017a). Nowadays awareness is increased by a lot of research and publications in the field of corrosion. Corrosion causing bacterial species and its interaction with metallic surfaces are more important for the development of effective inhibitor. Different types of inhibitors are available in cooling tower corrosion system in MIC like as chemicals (chlorine, chlorine dioxide, peracetic acid, quaternary ammonium, phosphates, benzotriazole, etc.) using high decimal ultrasound, rays like UV radiation, advanced oxidation process, electric charges and biological methods (green inhibitors such as each part of plants, see weeds) (Aruliah and Ting 2014).
The previous studies of green inhibitors are reported as organic compounds consist of nitrogen and sulphur to reduce the corrosion on the metal surface (Popoola 2019). Most organic inhibitors act as a protective layer of the metal surface by replaced water molecules. In the inhibitors, p electrons and lone pairs are present and they are helping to transfer the electrons between inhibitor and metal then develops a covalent bond and the cooling water system for different metals using various inhibitors was tabulated in Table 1. Green inhibitors are more efficient, economically profitable, high yield, no need to add chemicals (Chauhan and Gunasekaran 2007). The comparison of chemical inhibitors, biological inhibitors is the best way to reduce the MIC. Due to the chemical inhibitors, they cause pollution, non-biodegradable, harmful to humans and the environment (Chen et al. 2019).
Artemisia pallens are a short herb that belongs to the Asteracea family (Pavithra et al. 2018). It is important in the annual aromatic herb plant production of davana oil most popular in Indian country (Garai et al. 2012). A. pallens is a natural and non-toxic inhibitor and it has anti-microbial, anti-oxidant, fungicidal, anti-inflammatory, anti-tumor, and anti-malarial activities (Bouyanzer and Hammouti 2004). It contains 20% hydrocarbons, 65% ester and 15% oxygenated compounds such as arbutin, phenolic glycoside, germacranolides, davanone, pallensin and 4-epipallensin, artermone sesquiterpene ketone named as davanafurans and isodavanone, etc. (Garai et al. 2012; Kalaiselvi et al. 2010).
In the present study, methanolic extract of A. pallens (MEAP) used as a green inhibitor for inhibiting B. megaterium SKR7 corrosion causing bacteria and controls the MIC of mild steel 1010. The inhibition efficiency of MEAP was analyzed by biofilm assay and characterization of EPS studies. Biocorrosion of mild steel 1010 were analysis by weight loss (WL), electrochemical studies and Fourier transform infrared spectrum (FT-IR).
Materials and methods
Sample collection
Biofilm samples were collected on the metal surface which was used in a cooling tower plant placed in Ranipet tannery effluent treatment Limited, Ranipet, Tamil Nadu, India (latitude 12.9149857° and longitude 79.3459513°). The cooling tower tank capacity is 20–30 KL/day (kiloliters per day). In the cooling tower tank, reverse osmosis (RO) permeate reject water samples are used. The flow rate of water is 315-m cubic/h. The inlet temperature is 32–40 °C and outlet (vapor to cooling) 60–70 °C. The pH of the cooling tower water and chloride concentration was about 5.6 and 1.5%, respectively. The samples were collected from the above site and transferred to the lab with an ice-box and kept at 4 °C until further experiments.
Isolation of bacteria
The biofilm samples were subjected to serial dilution method and pour plate technique was employed using sterile nutrient agar for heterotrophic bacteria (HB), Iron agar for iron-oxidizing bacteria (IOB), Mn agar for manganese-oxidizing bacteria (MOB) and thiobacillus agar for acid producer bacteria (APB) isolation and the test was carried out in triplicate and further incubated at 37 °C for 24 h (Rajasekar et al. 2010). The total viable bacterial count was described earlier (Parthipan et al. 2017a) and the bacterial population was represented. The isolated colonies were selected randomly, and then purified colonies were streaked on the above said medium. Selected strains were preserved in sterile 50% glycerol for further experiments.
Identification of bacteria by 16S rDNA gene sequencing
The selected bacterial samples are sequenced in 16S rRNA as described earlier (Rajasekar et al. 2007b). In detail universal primers of forward and reverse 16S 5'-GGATGAGCCCGCGGCCTA-3' and Reverse Primer 5'-CGGTGTGTACAAGGCCCGG-3', respectively. Polymerase chain reaction (PCR) was carried out with a 100-μl mixture consist of 1 μl (57.9 ng) of DNA as the template, primer: forward-440 ng, reverse-440 ng, dNTPs (2.5 mM)-4 μl, 10 × Taq DNA polymerase assay buffer-10 μl, Taq DNA polymerase enzyme (3U/ml)-1 μl, water-80 μl (Biokart). Cycling conditions: initial denaturation (3 min at 94 °C), denaturation (1 min at 94 °C), annealing (1 min at 55 °C), extension (2 min at 72 °C), final extension (7 min at 72 °C) at 35 cycles. Sequencing reaction: 10 μl reaction mixture big dye terminator ready reaction mix with 4 μl, template (100 ng/ul) 1 μl, primer (10 pmol/λ), 2 μl, milli Q water, 3 μl PCR conditions: (25 cycles) initial denaturation: 96 °C for 5 min denaturation: 96 °C for 30 s hybridization: 50 °C for 30 s elongation: 60 °C for 1.30 min. Sequencing machine: ABI 3130 genetic analyzer. Chemistry cycle sequencing kit: big dye terminator version 3.1. Polymer and capillary array: POP_7 pol capillary array. Analysis protocol: BDTv3-KB-Denovo_v 5.2, data analysis: Seq Scape_ v 5.2, software reaction plate: Applied Biosystem Micro Amp, Optical 96-Well Reaction plate (Bruno et al. 2000). The obtained sequences were confirmed by BLAST searching tool.
Preparation of green biocorrosion inhibitor
A. pallens were collected from a local market in Vellore, Tamil Nadu. A. pallens was completely washed in sterile distilled water to remove the dust and soil and dried in a dark room at room conditions for 7 days. The dried leaves were grain into fine particles with the use of a mixer grinder. These fine powder particles are used as solvent extraction. 10 g of A. pallens powder was extracted in 100 ml of methanol using the Soxhlet apparatus (Kalaiselvi et al. 2010). Methanolic extract of A. pallens was dried using by vaporization and stored for further studies.
Minimal inhibition concentration (MIC) of methanolic extract of A. pallens against SKR7
The standard agar diffusion assay was used to determine the minimal inhibitory concentration of methanolic extract A. pallens against B. megaterium SKR7 at various concentrations (10, 25, 50, 75 and 100 ppm) (Parthipan et al. 2018).
Biofilm inhibition assay
Biofilm inhibition assay was followed by earlier methodology (Narenkumar et al. 2017a). In detail, the overnight bacterial culture of B. megaterium SKR7 was inoculated in the medium of nutrient broth (NB) and diluted in the ratio of 1:20 using sterile NB. 100 μl diluted bacterial cultures were transferred into the 96-well microtiter plate and the 25 ppm of MEAP was added and incubated at 37 °C for 24 h. After the incubation, plates were washed with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM NaHPO, 2 mM KHPO, pH 7.2) and 120 μl of crystal violet was added then incubated at 37 °C for 20 min. At the end, 125 μl of acetic acid was added on each well and incubated at 37 °C for 15 min. Visible changes in appearance were observed and to confirm the samples were analyzed by UV–visible spectrophotometer at 600 nm.
Estimation of extracellular polymeric substances
Overnight bacterial culture of Bacillus megaterium SKR7 in nutrient broth (NB) medium was diluted in a 1:20 ratio with fresh NB medium with and without 25 ppm of MEAP served as control and test samples and further incubated at 37 °C for 24 h. End of the incubation, bacterial culture was transferred into a centrifuge tube at aseptic condition and centrifuge at 5000 rpm for 45 min to obtain the supernatant solution. Further, an equal volume of isopropanol was added to the supernatant solution and kept at 8 °C for 12 h. After that, samples were centrifuge at 5000 rpm for 45 min to obtain the pellet and suspended with 0.1 M PBS (pH 7.2) for protein concentration and it was estimated by Lowry’s method (Narenkumar et al. 2107b).
Biocorrosion studies
Preparation of metal sample
The biocorrosion studies of mild steel (MS) 1010 were to assess weight loss (WL), electrochemical studies (ES) and Fourier transform infrared spectroscopy (FTIR). The configuration of mild steel 1010 is 0.03% C, 0.026% S, 0.01% P, 0.002% Si, 0.04% Ni, 0.002% Mo, 0.16% Mn, 0.093% Cu, and 99.64% Fe. The weight loss experiment procedure was described earlier by (Narenkumar et al. 2017a). MS 1010 coupons were cut with two size 2.5 cm2 for used weight loss experiment and 1 cm2 for used electrochemical studies and sequentially smoothened using a different grade of emery sheet finally rinsed with acetone and air-dried. 1 cm2 polished MS used as working electrode (WE) for EIS. The MS coupons were sterilized by the exposure of UV light prior to the biocorrosion experiment.
Weight loss measurement
MS coupons were used for biocorrosion studies by B. megaterium SKR7. Before the experiments, the initial weight of each coupon (in triplicate) was recorded. The experiment was carried out by four systems. System 1 (S1) as a control, it contains 400 ml of sterile cooling tower water (CTW) + 1% of nutrient broth (NB). System 2 (S2) contains 400 ml of CTW + 1% of NB + 1 ml of overnight grown B. megaterium SKR7 (2.2 × 106 CFU/ml). System 3 (S3) contains 400 ml of CTW + 1% of NB + 1 ml of B. megaterium SKR7 + 25 ppm of MEAP. System 4 (S4) contains 400 ml of CTW + 1% of NB + 25 ppm of MEAP. Three coupons were exposed in each system and were in triplicate. Finally, all systems were incubated for 20 days at room temperature (37 °C) in stagnant conditions. Corrosion coupons were removed and pickled (1 l HCl containing 20 g of antimony trioxide and 50 g of stannous chloride for 20 min) (Narenkumar et al. 2017c) for weight loss measurement (NACE 2005). The corrosion inhibition efficiency was calculated as per the following formula (Kalaiselvi et al. 2010).
where W and W1 are the weight loss of metal without and with inhibitors.
Surface analysis
Fourier transform infrared spectroscopy
After the incubation period of biocorrosion coupons were examined for surface analysis (Pavithra et al. 2018; Rajasekar et al. 2007a, b). The corrosion products were collected, dried and then crushed into a powder with potassium bromide (purified salt). The fine powder samples were analyzed for Fourier transform infrared spectroscopy (PerkinElmer Spectrum IR Version 10.6.0) examination of chemical compounds present in the coupons. It was analyzed in 400–4000 cm−1 wavenumbers at the resolution of 8 cm−1 and a scan rate of 64 scans/spectrum.
Electrochemical studies
Electrochemical analysis of Impedance spectroscopy was carried out by the Auto lab with Nova 2.1 software. MS1010 coupon of system 1, 2, 3, 4 (1 cm2) hired as a working electrode, saturated calomel electrode as a reference electrode and a platinum electrode as a counter electrode were used as impedance study. System 1, 2, 3, 4 liquid cultures are used for EIS studies in specific electrodes. Once a stable position was reached, an AC signal of 10 mV amplitude was supplied and impedance values were tested for frequencies varying from 0.1 Hz to 100 kHz. The Nyquist plot received the values of Rct. Polarization tests were performed potentiodynamically utilizing a Nova 2.1 program auto laboratory. The system was given 10 min to reach a constant potential performance it was carried out using different electrodes at a scan rate of 1800 mV/h from OCP + 200 mV SCE and −200 mV SCE. The analysis of polarization was performed on the 20th day of the time of immersion.
Results and discussion
Isolation of bacteria
Nine bacterial species were identified from biofilm samples by 16S rRNA sequencing as follows Bacillus sp., Pseudomonas sp., Staphylococcus sp., Pseudomonas sp., Cronobacter sp., (Rajasekar et al. 2007a). Among that B. megaterium has been selected for the biocorrosion studies. Neighbor-joining tree based on 16S rRNA gene sequences, showing phylogenetic in Fig. 1 (Bacillus related species). The phylogenetic analysis of the isolates presented the domination of Bacillus sp. in specific, B. megaterium SKR7 could be observed more dominant than other genera. 16S rRNA gene sequences of all bacterial species were submitted to NCBI and the GenBank accession numbers were presented in Table 2. Bacillus sp. was dominant group of corrosive bacteria among the isolated colonies from the cooling water system as described by Hussain et al. (2013). Hence, the B. megaterium SKR7 was selected for further studies.
Enumeration of total viable count
The total viable bacterial count (TVC) of a biofilm sample of heterotrophic bacteria was about 9.5 × 106 CFU/ml. The TVC of IOB, MnoB, and APB was a significantly increasing trend of about 104–106 CFU/ml. It reveals that bacterial isolated able to utilize the inorganic as a carbon source for their growth (Parthipan et al. 2017a). The selected bacterial isolate for further studies was a circular, convex, slightly yellow and gram-positive rod and identified as B. megaterium SKR7.
Minimal inhibition concentration (MIC)
The MIC of MEAP for the inhibition of B. megaterium SKR7 was evaluated by the agar diffusion method (Parthipan et al. 2018). The diameter of zone of inhibition can determine the corrosion inhibition by the extract. From the results reported 25 ppm of MEAP shows the 3.07 ± 0.1 zone of inhibition as optimum concentration. Further, positive control of ampicillin was to obtain the bacterial inhibition zone of 2.26 ± 0.1 were, respectively. The present study revealed that the extraction of biological compound (inhibitor) from MEAP to exhibit the excellent antibacterial properties against cooling water corrosion causing bacterial strain.
Biofilm inhibition assay
Biofilm formulation was studied with a crystal-violet by 96 well microtiter plate assay described earlier (Narenkumar et al. 2017a). The formation of biofilm layer is shown in B. megaterium SKR7 (S2) well by observing the presence of a dark violet colour (Fig. 2) and S2 were more actively attached to the surface of the metal. In addition to MEAP, the reduced biofilm formation (S3) was observed as light in colour which indicated that MEAP act as an efficient antibacterial activity.
Extracellular polymeric substance (EPS) analysis
EPS analysis of B. megaterium SKR7 with 25 ppm of MEAP was estimated by Lowry’s method. The estimation of protein and carbohydrate content was gradually reduced, which was compared to with and without MEAP. The higher amount of proteins (0.045 mg/ml) and carbohydrate (0.022 mg/ml) were observed in S2 (B. megaterium SKR7). The addition of inhibitor S3 (B. megaterium SKR7 + MEAP) values were decreased for protein (0.009 mg/ml) and carbohydrate (0.002 mg/ml), respectively. In conclusion, MEAP compound had the excellent potency of antimicrobial activity and reduces the extracellular polymeric substance and thus prevent the biofilm formation.
Weight loss measurement
Weight loss measurement of mild steel (MS 1010) coupons of systems was presented in Table 3. In the S1, an average weight loss was recorded at 0.024 ± 0.1 mg, whereas in experimental S2 and S3 0.046 ± 0.1 and 0.008 ± 0.1, respectively. The corrosion rate of S1 was slightly higher corrosion rate (0.8009 mm/year) and S2 and S3 represent 1.5350 and 0.2669 mm/year. Its confirmed MEAP can inhibit the corrosion causing bacterial growth of cooling tower. S4 indicates the WL 0.010 ± 0.1, CR 0.0.3335 was significantly reduced in the presence of MEAP due to its corrosion inhibition property (Bouyanzer and Hammouti 2004). The pitting corrosion was observed due to the oxidation and reduction reaction of the metal surface (Eqs. 1, 2). The corrosion inhibition efficiency of MEAP with and without SKR7 was 83% and 58%, respectively.
Surface analysis
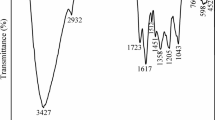
FTIR analysis of corrosion samples showed a different peak and indicates that different functional groups present on the metal surface (Fig. 3). These functional groups represent the 684.48 cm−1 was =C–H strong broad bending (alkynes), peak at 1070.50 cm−1 indicates the C–O stretching for alcohols in an antimicrobial compound, 1384.01 cm−1 represents CH3C–H bending in both alkanes and alkyls have metal chelant with organic acids, 1641.35 cm−1 C=O stretching (amides) with industrial catalyst activity, and 3463.56 cm−1 showed a strong and broad O–H stretch (alcohol) activity of flavoring agent. S3 and S4 represent the =C–H strong broad bending alkynes and C–O stretching (alcohols) are changed due to the metal and bacterial interaction. In system S3, the functional groups indicate the inhibition of bacterial growth. Results of FTIR concluded that oxidation and reduction reaction takes place in presence of MEAP with B. megaterium on metal as a result from the inhibition of biofilm which was observed in the changes of functional groups of FTIR spectrum (Pavithra et al. 2018).
MEAP follows the Langmuir’s adsorption isotherm theory on the metal surface and increases the activation energy of corrosion inhibition process through interaction between arbutin compound (donor) which has the functional group OH group (N, O, and aromatic rings). These groups indicate the presence of phenolic molecules in MEAP which also consists of π electron atoms and the vacant of d-orbital (acceptor) of iron in the metal surface were binds and leads to form a defense complex as a resulted decreases in corrosion process (Garai et al. 2012). The phenyl ring increased electron density as well as increase their adsorption rate (Yadav et al. 1999) and hydroxyl groups alter each molecule of living cells by caused damaging the DNA strands (Bora and Sharma 2011). Kalaiselvi et al. 2010 reported the aromatic nitrogen-based compounds are present in the MEAP responsible for the corrosion inhibition. The strong multi-bands bonded N–H stretch of a functional group of aromatic and heteroaromatic and C–N stretching of amidines. Generally, the presence of nitrogen compounds acts as an inhibitor for biological metabolism. The natural inhibitors have electroactive aromatic nitrogen rings for adsorption of the molecules and form the protective metal surface coatings (Rani and Basu 2012; Bensouda et al. 2018). As reported by (Bail et al. 2008) MEAP consist of the functional group of cis-davanone which was a most essential compound with antimicrobial activity and also a trace amount of bicyclogermacrene, linalool, caryophyllene oxide, phytol and also derivatives of ethers. (Pavithra et al. 2018) also reported that MEAP has anti-microbial and anti-viral activity with the constituents of isophytol compounds.
Electrochemical studies
Potentiodynamic Tafel polarization
The corrosion current (icorr) of the S1 was about 13.93 μA/cm2, whereas in the presence of S2 B. megaterium values were higher about 19.88 μA/cm2. In the presence of MEAP S3 and S4 the icorr was lower about 8.02 μA/cm2 and S4 4.09 μA/cm2, (Table 4; Fig. 4), respectively. It revealed that MEAP act as a good inhibitor as well as inhibition of biofilm. Due to this experiment of S2, the peak was larger in the anodic side and represented the increase in potential values resulted as icorr increases which leads to the formation of pitting corrosion. In system S3, MEAP could be reduced the corrosion density and inhibit the corrosion reaction. Due to the presence of an arbutin compound, it was absorbed by the metal surface and forms a protective layer and the arbutin compound transfer the π electron to metal outer area and thus inhibition complex (Kalaiselvi et al. 2010).
Impendence analysis
The impedance studies were shown in Table 5 and Fig. 5. The charge transfer resistance (Rct) of the S1 was about 27 Ω cm2, on the other hand in the presence of S2 B. megaterium values was decreased about 24 Ω cm2. In the presence of MEAPS3 and S4 systems Rct value was totally increased 34 Ω cm2 and S4 50 Ω cm2, respectively. S2 can form the thick biofilm layer so the current observation is low and S3 and S4 (added MEAP) prevent the biofilm formation because MEAP charge of the electrons, the electrode can be moved from the non-aqueous phase (electrode) to the aqueous phase and develop metal protective layer that can be resistant to bind the microbes. In the presence of arbutin chemical compounds is adsorb to the mild steel surface (Garai et al. 2012).
Conclusion
The current investigation was reported the role of MEAP as a natural green corrosion inhibitor against the biocorrosion of mild steel 1010 by B. megaterium. MEAP consists of aromatic nitrogen-based compounds and it was formed as a film on the metal surface and thus inhibits the bacterial adsorption, which was confirmed by the weight loss, EIS experiments. EIS confirms the B. megaterium forms pitting corrosion of metal. As a result of these studies, 25 ppm of MEAP was identified as optimal dosages for the biofilm inhibition of mild steel surface and acted as an anodic type of inhibitor with the inhibition of 83%.
References
Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib NE (2014) Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch Microbiol 196(7):453–472. https://doi.org/10.1007/s00203-014-0983-1
Ahmed SA, Makki HF (2020) Corrosion behavior of mild-steel in cooling towers using high salinity solution. In: AIP Conference Proceedings 2213(1):020178. AIP Publishing LLC. doi: https://doi.org/10.1063/5.0000274
Aruliah R, Ting YP (2014) Characterization of corrosive bacterial consortia isolated from water in a cooling tower. ISRN Corrosion, Article ID 803219, p.11. doi: https://doi.org/10.1155/2014/803219
Atalah J, Blamey L, Köhler H, Alfaro-Valdés HM, Galarce C, Alvarado C, Sancy M, Páez M, Blamey JM (2020) Study of an Antarctic thermophilic consortium and its influence on the electrochemical behavior of aluminum alloy 7075–T6. Bioelectrochemistry 133:107450. https://doi.org/10.1016/j.bioelechem.2019.107450
Bail S, Buchbauer G, Schmidt E, Wanner J, Slavchev A, Stoyanova A, Denkova Z, Geissler M, Jirovetz L (2008) GC-MS-analysis, antimicrobial activities and olfactory evaluation of essential davana (Artemisia pallens Wall. ex DC) oil from India. Nat Prod Commun 3(7). doi: https://doi.org/10.1177/1934578X0800300705
Bensouda Z, Elassiri E, Galai M, Sfaira M, Farah A, Touhami ME (2018) Corrosion inhibition of mild steel in 1 M HCl solution by Artemisia Abrotanum essential oil as an eco-friendly inhibitor. J Mater Environ Sci 9:1851–1865. https://doi.org/10.26872/jmes.2018.9.6.205
Bentham RH, Broadbent CR (1995) Field trial of biocides for control of Legionella in cooling towers. Curr Microbiol 30(3):167–172. https://doi.org/10.1007/BF00296203
Bora KS, Sharma A (2011) Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm Biol 49(12):1216–1223. https://doi.org/10.3109/13880209.2011.578142
Bouyanzer A, Hammouti B (2004) A study of anti-corrosive effects of Artemisia oil on steel. Pigm Resin Technol. https://doi.org/10.1108/03699420410560489
Bruno WJ, Socci ND, Halpern AL (2000) Weighted neighbor joining: a likelihood-based approach to distance-based phylogeny reconstruction. Mol Biol Evol 17(1):189–197. https://doi.org/10.1093/oxfordjournals.molbev.a026231
Chauhan LR, Gunasekaran G (2007) Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros Sci 49(3):1143–2116. https://doi.org/10.1016/j.corsci.2006.08.012
Chen C, Wang Y, Liu S, Feng R, Gu X, Qiao C (2019) Research on the application of compound microorganism preparation in reusing urban reclaimed water in circulating cooling water system. Water Sci Technol 80(9):1763–1773. https://doi.org/10.2166/wst.2019.430
Chirkunov A, Kuznetsov Y (2015) Corrosion inhibitors in cooling water systems. In: Amjad Z, Demadis KD (eds) Mineral scales and deposits: scientific and technological approaches. Elsevier, pp 85–105
Dhaidan SK, Reshan SAA, Hussain MS (2009) Assessment of phenol derivatives in cooling tower system as biocides. J Eng 15(4):4429–4437
Ford TE, Black JP, Mitchell R (1990) Relationship between bacterial exopolymers and corroding metal surfaces. Corrosion 90/110 NACE Houston
Garai S, Garai S, Jaisankar P, Singh JK, Elango A (2012) A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros Sci 60:193–204. https://doi.org/10.1016/j.corsci.2012.03.036
Garrett JH (1891) The action of water on lead, etc. HK Lewis, London
George RP, Mudali UK, Raj B (2016) Characterizing biofilms for biofouling and microbial corrosion control in cooling water systems. Anti-Corros Method M. https://doi.org/10.1108/ACMM-07-2014-1401
Gogoi PK, Barhai B (2010) Corrosion inhibition of carbon steel in open recirculating cooling water system of petroleum refinery by a multi-component blend containing zinc (II) diethyldithiocarbamate. https://nopr.niscair.res.in/handle/123456789/10000
Hsieh MK, Li H, Chien SH, Monnell JD, Chowdhury I, Dzombak DA, Vidic RD (2010) Corrosion control when using secondary treated municipal wastewater as alternative makeup water for cooling tower systems. Water Environ Res 82(12):2346–2356. https://doi.org/10.2175/106143010x12681059117094
Kalaiselvi P, Chellammal S, Palanichamy S, Subramanian G (2010) Artemisia pallens as corrosion inhibitor for mild steel in HCl medium. Mater Chem Phys 120(2–3):643–648. https://doi.org/10.1016/j.matchemphys.2009.12.015
Moura V, Ribeiro I, Moriggi P, Capão A, Salles C, Bitati S, Procópio L (2018) The influence of surface microbial diversity and succession on microbiologically influenced corrosion of steel in a simulated marine environment. Arch Microbiol 200(10):1447–1456. https://doi.org/10.1007/s00203-018-1559-2
NACE R (2005) 0775 “Preparation installation, analysis, and interpretation of corrosion coupons in oilfield operation.” NACE International: standard recommended practice
Narenkumar J, Parthipan P, Nanthini AUR, Benelli G, Murugan K, Rajasekar A (2017a) Ginger extract as green biocide to control microbial corrosion of mild steel. 3 Biotech 7(2):133. https://doi.org/10.1007/s13205-017-0783-9
Narenkumar J, Sathishkumar K, Sarankumar RK, Murugan K, Rajasekar A (2017b) An anticorrosive study on potential bioactive compound produced by Pseudomonas aeruginosa TBH2 against the biocorrosive bacterial biofilm on copper metal. J Mol Liq 243:706–713. https://doi.org/10.1016/j.molliq.2017.08.075
Narenkumar J, Sathishkumar K, Selvi A, Gobinath R, Murugan K, Rajasekar A (2017c) Role of calcium-depositing bacteria Agrobacterium tumefaciens and its influence on corrosion of different engineering metals used in cooling water system. 3 Biotech 7(6):374. https://doi.org/10.1007/s13205-017-1007-z
Narenkumar J, AlSalhi MS, Arul Prakash A, Abilaji S, Devanesan S, Rajasekar A, Alfuraydi AA (2019a) Impact and role of bacterial communities on biocorrosion of metals used in the processing industry. ACS Omega 4(25):21353–21360. https://doi.org/10.1021/acsomega.9b02954
Narenkumar J, Elumalai P, Subashchandrabose S, Megharaj M, Balagurunathan R, Murugan K, Rajasekar A (2019b) Role of 2-mercaptopyridine on control of microbial influenced corrosion of copper CW024A metal in cooling water system. Chemosphere 222:611–618. https://doi.org/10.1016/j.chemosphere.2019.01.193
Parthipan P, Babu TG, Anandkumar B, Rajasekar A (2017a) Biocorrosion and its impact on carbon steel API 5LX by Bacillus subtilis A1 and Bacillus cereus A4 isolated from Indian crude oil reservoir. J Bio Tribo Corros 3(3):32. https://doi.org/10.1007/s40735-017-0091-2
Parthipan P, Narenkumar J, Elumalai P, Preethi PS, Nanthini AUR, Agrawal A, Rajasekar A (2017b) Neem extract as a green inhibitor for microbiologically influenced corrosion of carbon steel API 5LX in a hypersaline environment. J Mol Liq 240:121–127. https://doi.org/10.1016/j.molliq.2017.05.059
Parthipan P, Elumalai P, Narenkumar J, Machuca LL, Murugan K, Karthikeyan OP, Rajasekar A (2018) Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int Biodeter Biodegr 132:66–73. https://doi.org/10.1016/j.ibiod.2018.05.005
Pavithra KS, Annadurai J, Ragunathan R (2018) Phytochemical, antioxidant and a study of bioactive compounds from Artemisia pallens. J Pharmacogn Phytochem 7(4):664–675
Popoola LT (2019) Organic green corrosion inhibitors (OGCIs): a critical review. Corros Rev 37(2):71–102
Preethi PS, Narenkumar J, Prakash AA, Abilaji S, Prakash C, Rajasekar A, Nanthini AUR, Valli G (2019) Myco-synthesis of zinc oxide nanoparticles as potent anti-corrosion of copper in cooling towers. J Clust Sci 30(6):1583–1590. https://doi.org/10.1007/s10876-019-01600-0
Rahmani K, Jadidian R, Haghtalab S (2016) Evaluation of inhibitors and biocides on the corrosion, scaling and biofouling control of carbon steel and copper–nickel alloys in a power plant cooling water system. Desalination 393:174–185. https://doi.org/10.1016/j.desal.2015.07.026
Rajasekar A, Ting YP (2011) Role of inorganic and organic medium in the corrosion behavior of Bacillus megaterium and Pseudomonas sp. in stainless steel SS 304. Ind Eng Chem Res 50(22):12534–12541. https://doi.org/10.1021/ie200602a
Rajasekar A, Rajendran L, Maruthamuthu S, Palaniswamy N, Rajendran A (2006) Prediction of corrosion rate of steel AP5LX using curve fitting method. Zaštitamaterijala 47(4):47–50
Rajasekar A, Maruthamuthu S, Palaniswamy N, Rajendran A (2007a) Biodegradation of corrosion inhibitors and their influence on petroleum product pipeline. Microbiol Res 162(4):355–368. https://doi.org/10.1016/j.micres.2006.02.002
Rajasekar A, Ponmariappan S, Maruthamuthu S, Palaniswamy N (2007b) Bacterial degradation and corrosion of naphtha in transporting pipeline. Curr Microbiol 55(5):374–381. https://doi.org/10.1007/s00284-007-9001-z
Rajasekar A, Anandkumar B, Maruthamuthu S, Ting YP, Rahman PK (2010) Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol 85(4):1175–1188. https://doi.org/10.1007/s00253-009-2289-9
Rani BE, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Intern J Corros. https://doi.org/10.1155/2012/380217
Rochdi A, Kassou O, Dkhireche N, Touir R, El Bakri M, Touhami ME, Sfaira M, Mernari B, Hammouti B (2014) Inhibitive properties of 2, 5-bis (n-methylphenyl)-1, 3, 4-oxadiazole and biocide on corrosion, biocorrosion and scaling controls of brass in simulated cooling water. Corros Sci 80:442–452. https://doi.org/10.1016/j.corsci.2013.11.067
Rodriguez-Leiva M, Tributsch H (1988) Morphology of bacterial leaching patterns by Thiobacillusferrooxidans on synthetic pyrite. Arch Microbiol 149(5):401–405. https://doi.org/10.1007/bf00425578
Matt Schnepf. (2018). Identifying types of corrosion in water systems. Retrieved May 2020. https://www.chemaqua.com/en-us/Blog/identifying-types-of-corrosion-in-water-systems-1
Selvi A, Ananthaselvam A, Narenkumar J, Prakash AA, Madhavan J, Rajasekar A (2019) Effect of nano-zerovalent iron incorporated polyvinyl-alginate hybrid hydrogel matrix on inhibition of corrosive bacteria in a cooling tower water environment. SN Appl Sci 1(5):424. https://doi.org/10.1007/s42452-019-0443-2
Shi X (2010) On the use of nanotechnology to manage steel corrosion. Recent Pat Eng 4(1):44–50. https://doi.org/10.2174/187221210790244730
Soares SS, Souza TK, Berté FK, Cantarelli VV, Rott MB (2017) Occurrence of infected free-living amoebae in cooling towers of Southern Brazil. Curr Microbiol 74(12):1461–1468. https://doi.org/10.1007/s00284-017-1341-8
Telegdi J, Shaban A, Trif L (2020) Review on the microbiologically influenced corrosion and the function of biofilms. Int J Corros Scale Inhib 9(1):1–33. https://doi.org/10.17675/2305-6894-2020-9-1-1
Tewari R, Mehta SK, Vaishnav P (2019) Influence of microbes in cooling tower: a review. Int Res J Eng Technol 6(8):376–383
Xu P, Xu Z, Wang J, Zhang Y, Liu T (2012) Microbiological induced corrosion on brass in recycling cooling water system makeup by reclaimed water. Mat Sci Appl 3(4):253–258. https://doi.org/10.4236/msa.2012.34037
Yadav PNS, Singh AK, Wadhwani R (1999) Role of hydroxyl group in the inhibitive action of benzoic acid toward corrosion of aluminum in nitric acid. Corrosion 55(10):937–941. https://doi.org/10.5006/1.3283929
Zadvorny OA, Zorin NA, Gogotov IN (2006) Transformation of metals and metal ions by hydrogenases from phototrophic bacteria. Arch Microbiol 184(5):279–285. https://doi.org/10.1007/s00203-005-0040-1
Zobell CE (1943) The effect of solid surfaces upon bacterial activity. J bacterial Res 46(1):39
Acknowledgements
The authors are grateful to the researchers supporting project number (RSP-2020/68), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors listed in the manuscript declare no conflict of interest in this publication. The tannery cooling tower water and biofilm samples were collected from the authorities of Ranipet tannery effluent treatment Co. Ltd., Tamil Nadu, India with proper permission.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kokilaramani, S., AlSalhi, M.S., Devanesan, S. et al. Bacillus megaterium-induced biocorrosion on mild steel and the effect of Artemisia pallens methanolic extract as a natural corrosion inhibitor. Arch Microbiol 202, 2311–2321 (2020). https://doi.org/10.1007/s00203-020-01951-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01951-7