Abstract
Oral lichen planus (OLP) is a relatively common chronic inflammatory disease. The micronutrients are critical factors in health of oral mucous and proper function of immune system. There have not been any review articles for evaluating trace element levels before and after standard treatments of OLP. The purpose of this study is to provide complete review of the association of micronutrients with OLP. Databases including PubMed, Google Scholar, Scopus, and Embase (Ovid) with keywords of oral lichen planus, OLP, oral disorder, micronutrients, trace element, nutrient element, antioxidant, oxidative stress, malnutrition, and essential trace elements, without time limitation (1900–2019) were searched to collect data on related articles. Total number of 58 original articles including 12 randomized clinical trials, 41 case-control, 4 case reports, and 1 cell line research were reviewed in this study. Lower levels of iron and its associated markers, such as hemoglobin and ferritin, increased levels of TIBC; reduced levels of zinc, calcium, vitamin D, vitamin B12, folic acid, and antioxidants such as vitamins C and E; and increased levels of oxidants and homocysteine, have been reported in OLP patients.
Similar content being viewed by others
Introduction
Micronutrients, including trace elements, vitamins, and antioxidants, play an important role in regenerative processes against oxidative stress products in the tissues. The clinical features of micronutrient malnutrition on the oral health mucosa are widely recognized, comprising immune system complication and susceptibility to various oral and systemic diseases [1]. Trace elements are found in low amounts in natural environments [2]. According to the WHO classification, trace elements have been divided into three groups: Essential trace elements (ETE), probably essential elements, and potentially toxic elements [3]. Each trace element is related to the functions of a lot of enzymes, a combination of various symptoms is related to the deficiency of a single trace element rather than any specific clinical manifestations [1]. Vitamins are organic essential micronutrients that an organism needs in small quantities for the proper functioning of its metabolism; some of them have hormonal activity [4, 5]. Oxidant-antioxidant imbalance resulting in excessive accumulation of ROS is defined as oxidative stress [6]. Many investigators have noticed the association between micronutrient deficiency and inflammatory disorders. Lichen planus (LP) is a chronic inflammatory disease accompanied by several clinical manifestations that affect the skin, nails, hair, and mucous membranes [7]. The mechanism of T cell-mediated apoptosis depends on the expression of an unknown antigen that inactivates keratinocytes [8]. Adequate intakes of micronutrients are required for the efficient function of the immune system. Micronutrient deficiency dysregulates the balanced host natural defense response by suppressing immune functions through affecting the innate (physical barriers in skin/mucosa), cellular, and humoral immunity systems [9].
As there had been limited knowledge among the oral physicians regarding significance of trace elements in OLP patients, the current review focuses on the role of those micronutrients including essential trace elements, vitamins, and antioxidants, which have a proven role in maintaining oral health and their implications in OLP.
Materials and Methods
Databases including PubMed, Google Scholar, Scopus, Web of Science (SCI), and Embase with search terms of “lichen planus” OR “oral lichen planus” OR “OLP” AND, “oral disorder’ OR “Oral diseases”, AND,” micronutrients” OR” nutrient element” AND “trace element” OR “essential trace elements”, AND, “antioxidant’, AND, “oxidative stress”, AND, “malnutrition”, without time limitation (1900–2019) were searched to collect data on related articles. The inclusion criteria were original article including case-control, case report, case series and clinical trials, evaluation of one of the micronutrients in OLP patients, confirmation of OLP according to clinical and histopathologic criteria based on modified WHO or krunch-koff and Eisenberg criteria, measurement of one of the micronutrients levels in at least one medium such as saliva, serum, transudate/tissue specimens or cell line, interventional studies to evaluate the efficacy of micronutrients in the management of OLP also evaluated the lesion response, and articles published only in the English language.
Exclusion criteria comprised of reviews, abstracts, commentaries, letters to the editor, opinion articles, review articles, evaluation toxic elements, other oral disorder, oral lichenoid reactions instead of OLP, or diagnosed OLP according to no reliable criteria or showing evidence of dysplasia of the tissue.
Results
Two authors (N.GH. and N. SH.) independently searched the above databases and assessed the titles and abstracts of all eligible publications.
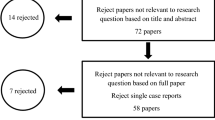
By searching the mentioned databases, 467studies were found. Then we removed the duplicate articles. A total of 122 articles related to the subject were identified and their full text was extracted in PDF format. Of these, 64 articles were excluded due to inconsistency with the inclusion criteria. Finally, 58 completely related articles that met the inclusion criteria were included in this study.
Micronutrients in patients have been studied in three main categories: trace elements, vitamins, and antioxidants. Measurements are often taken directly in different mediums such as a serum, saliva, urine, and tissue samples, while in some studies, indirectly, micronutrient-dependent mediators have been studied, for example, vitamin D receptor (VDR) levels are associated with vitamin D or antibodies against gastric parietal cells associated with vitamin B12 levels. Genetic studies in this area appear to have a very small percentage, some of which have studied and compared single nucleotide polymorphisms in genes associated with micronutrients in patients and healthy individuals, or overexpression or underexpression of microRNAs associated with pathogenesis in OLP after treatment with some micronutrients. A relatively large group of studies have also investigated the therapeutic effects of various micronutrients in improving or reducing the symptoms of OLP patients. Among the 12 clinical trial studies that examined the therapeutic effects of micronutrients, 5 of them were before and after and 6 studies were randomized clinical trials. Three case report studies have also examined the therapeutic effects of micronutrients in patients with OLP. One study also looked at the therapeutic effects of vitamin A on the cell lines involved in OLP. Most interventional studies have examined the severity of OLP clinical symptoms in most cases based on the visual analogue scale (VAS) and Thongprasom criteria, and some have considered other levels of markers or inflammatory indicators. Case-control studies are the largest category of studies. In some cases, tissue or cellular samples have been compared, and in others, serum, salivary, or urinary levels of micronutrients have been compared (Tables 1, 2, 3, 4, and 5).
Discussion
According to the best of our knowledge, serum is the most common medium for examining levels of micronutrients in OLP patients. All three major groups of micronutrients have been studied in serum. Of these, zinc and iron are the most common trace elements studied in OLP. All researches about folic acid and vitamin B12, except for one clinical trial study, have been performed on serum as case-control studies. However, only one study examined vitamin A levels in serum. Two studies have reported a higher prevalence of polymorphisms in genes associated with micronutrients such as VDR and methylenetetrahydrofolate reductase (MTHFR) 677 in OLP patients (Table 1). Saliva has also been a popular medium in recent years for examining many markers associated with various diseases. Of the nine studies that evaluated salivary levels of micronutrients, seven of them reported increased levels of antioxidant, and two reported decreased in trace element levels in OLP patients (Table 2). Two studies have examined the serum and salivary levels of micronutrients at the same time. Ergun et al. showed a significant correlation between oxidant and antioxidant salivary and serum levels, and Chuykin showed similar results between trace elements in saliva and serum of patients (Table 3). These results suggest that saliva can be considered as a reliable medium in assessing micronutrient levels instead of serum in the future. More accessibility, more accurate, less expensive, and less risk of transmission of infection to healthcare workers and cross infection reduce the stress and discomfort of patients in saliva sampling to serum.
Based on the results of clinical trials in the vast majority of these studies, improvement of clinical symptoms and decrease pain, burning sensation, and size of the lesions using VAS and Thongprasom criteria have been reported to be significant. Also, some interventional studies have shown a significant decrease in the level of markers or inflammatory indicators. It should be noted that most of these indicators have been studied in patients’ serum and only one case as a cell line. Vitamin A and antioxidants such as vitamin C and selenium are the most common micronutrients that have been shown to have therapeutic effects in OLP patients. According to the results of the research, the only trace element that has proven therapeutic effects in OLP patients is zinc, which shows the importance of examining the therapeutic effects of other micro-neutrinos in future studies (Table 4). In other mediums such as urine, direct or indirect tissue specimen’s analysis, and cellular examination, a significant reduction in the number of micronutrients associated with OLP has been confirmed (Table 5).
Micronutrients are one of the cornerstones in the maintenance of biodynamic of the body. Both deficiency and excess of micronutrients trigger and continue the progression of various diseases [10] by impairment of critical mechanisms of the host defense including cellular membrane stability, apoptosis, host metabolism, and enzymatic activity [9]. The clinical interest in micronutrient determination for the diagnosis and treatment of different diseases has increased in recent years [3]. Of the trace elements, six of them have been studied more in patients with OLP.
Zinc has vital roles in cellular growth and division, normal immune function, collagen synthesis, and wound healing [4]. Inadequate zinc intake through disturbance in innate cellular immunity, Th1 response, and maintenance of skin and mucosal membrane disintegrate is associated with the pathogenesis of lichen planus [11,12,13,14].
Most of the included articles in this study indicated a significant decrease in serum and/or salivary levels of zinc in the OLP patients (Tables 1, 2, and 3). In addition, the decrease in zinc levels in OLP women appears to be more pronounced than in OLP men [15]. In contrast, two studies reported increased zinc levels in OLP patients [16, 17]. In spite of reporting a significant increase in the zinc salivary level in patients with pre-malignant and malignant lesions compared to the control group, they found that the copper/zinc ratio was decreased in both groups compared to the control group [16]. Another research has also suggested the importance of zinc deficiency in increasing the risk of oral cavity malignancy [12]. In addition, topical and systemic forms of zinc resulted in a significant improvement of OLP lesions [18, 19].
Calcium (Ca) acts as an intracellular mediator in regulating the immune system function such as differentiation of immune cells and gene transcription [10]. Hypocalcemia has been reported as the causative factor in oral ulcers such as aphthous stomatitis [20]. We found some controversies in reviewed articles in regard to calcium level in OLP patients. Ma et al. showed that a decrease in Ca levels in peripheral blood lymphocytes of OLP patients was associated with a significant increase in the expression of STM1 and MBP-Golli mRNA, which was effective in T-lymphocyte activity and function [21]. In one study [17], the salivary Ca levels, although insignificant, were higher than in the control group.
Iron is necessary for cellular differentiation and proliferation. Iron deficiency causes a thin layer of epithelium, mucosal inflammatory, and atrophy changes that expose mucosa to environmental factors impairing the immune system and increasing permeability [9]. Based on our research, serum and salivary levels of iron and related markers such as ferritin and hemoglobin in the OLP patients were significantly lower than in healthy subjects (Tables 1, 2, and 3). The prevalence rate of OLP in IDA patients is significantly higher than in healthy individuals [22]. CHANG et al. showed that the presence of autoantibodies against parietal and thyroid cells in the OLP patients was significantly correlated with decreased iron and hemoglobin levels [23,24,25]. Adjuvant therapy with topical corticosteroid and iron supplementation resulted in significant improvement of OLP lesions. With iron deficiency, the turnover of epithelial cells becomes faster and causes atrophic or immature mucosa [22]. In addition, aging due to low-grade inflammation in the body is associated with impaired iron absorption and metabolism, and ultimately a decrease in iron stores in the body, which can predispose the oral mucosa to diseases such as OLP at an older age [26].
Copper contributes to the proper function of the immune system components such as neutrophils, monocytes, and superoxide dismutase. Copper along with catalase and glutathione peroxidase is involved in the cytosolic antioxidant defense system against ROS. Excessive or insufficient amounts of copper affect the immune system in the long term [9]. There are controversies about the level of copper in OLP patients (Tables 1, 2, and 3). Ayinampudi et al. stated that the reduction of the copper/zinc ratio in pre-malignant and malignant lesions was more important than the levels of each of these elements separately [16]. In pre-malignant lesions, such as OLP, elevated oxidative stress levels established may justify an increase in copper levels in response to oxygen free radicals in these patients [26].
Magnesium (Mg) plays an important role in cellular membrane stabilization and neural conduction and enzymes function [27]. There are several controversies about magnesium levels in OLP patients in various studies. In one study a significant decrease in salivary Mg levels in OLP patients has been reported, it seems that this finding is related to increased macrophage and neutrophil activity, autoimmune processes, and free radical oxidation [17]. However, salivary and serum Mg levels in the OLP patients in another study showed higher levels than the control; also, the severity of lesions had a direct association with increasing Mg level. This increase is likely a reflection of the nonspecific compensatory response to decrease inflammatory responses [27].
Selenium accompanied by other antioxidants such as vitamins A, C, and E is a crucial factor in proper innate and acquired immune responses. It triggers the expression of the genes encoding the proteins involved in immune response (cytokines and adhesion molecules). Selenium deficiency reduces immunoglobulin titer and cellular immunity [28]. We found two articles that evaluated the therapeutic effects of selenium on OLP patients. Belal et al. (2015) showed that a combination of SE-ACE (selenium combined with vitamins A, C, and E), corticosteroids, and antifungals is effective in treating erosive OLP but further studies are recommended with more sample size and longer assessment period [29]. H Wu et al. showed that selenium is effective in treating OLP patients by regulating CD3+/CD4+ and CD4+/CD8+ expressions [30].
In addition to trace elements, other groups of micronutrients are vitamins. Vitamin B12 acts as an immunomodulator for cellular immunity, especially in CD8+ and NK cells [31]. Three categories of studies were found to evaluate vitamin B12 levels in OLP patients.
The first group examined serum and/or salivary levels of vitamin B12. Most studies reported a significant or insignificant decrease in this vitamin level in the OLP patients (Tables 1, 2, 3, and 4). In a study, a higher percentage of vitamin B12 depletion in men than in women (50.5/21.5%) probably reflects a more pronounced role of this vitamin in the pathogenesis of OLP in men. Besides, vitamin B12 deficiency has been linked to increased lesion severity and the risk of dysplasia [31]. The second set of studies examined the levels of antibodies (Ab) against parietal cells and the presence of pernicious anemia in the OLP patients [32]. These studies showed that levels of this Ab were significantly increased in the OLP patients and administration of vitamin B12 alone or in combination with immunomodulatory drugs could be effective in decreasing the levels of autoantibodies, pain, and severity of lesions in OLP [32, 33]. The third group of studies simultaneously examined levels of B12 and auto-Ab against parietal cells and MCV [23,24,25]. In all of these studies, elevated levels of auto-Ab and MCV have been reported to coincide with a decrease in B12 levels. Accordingly, it is recommended to check the level of B12 and other auto-Ab levels. It is noteworthy that the administration of an intramuscular drug (IM) is preferred over the oral form in patients with P.A due to the absence of an intrinsic factor necessary for vitamin B12 uptake [33]. The levels of vitamin B12 and folic acid have been co-evaluated in most studies. Since lowering serum B12 levels disrupts folate metabolism and results in lower levels, folic acid assays are strongly recommended in patients with B12 deficiency [31]. Some researchers believe that the presence of erosive OLP (EOLP) lesions due to increased irritation and pain during chewing leads to malnutrition and exacerbation of decreased folic acid and B12 levels in patients. However, others have suggested similar effects of folic acid on vitamin B12 through neuropsychological disorders in the etiology of OLP [31]. Of the other group B vitamins, vitamins B1 and B6 have been evaluated in the OLP patients, which showed no significant difference [34].
Folic acid is a key factor in T cell-mediated apoptosis. Folate deficiency reduces the circulating number of T-lymphocytes and their proliferation, innate immune responses, and resistance against infections in the elderly [35]. Folic acid along with vitamin B12 is essential for cell growth and body metabolism. Our study generally showed a decrease in folic acid levels in the OLP patients significantly or non-significantly (Tables 1, 2, 3, and 4). Nosratzadeh and Jolly showed folic acid administration to be effective in treating cutaneous or oral lichen planus by regulating immune system activity they reported that although hematologic deficiencies are higher in OLP patients than in healthy subjects, they may not be the main etiology of OLP [4, 36]. However, some studies have considered folic acid depletion through the development of depression and psychologic stresses as an important etiologic agent in the incidence of OLP [37].
Homocysteine is a potent stimulator of cellular activity and differentiation [38]. A decrease in the level of folic acid and vitamin B12 increases the concentration of homocysteine in patients with Alzheimer’s disease (AD) and rheumatoid arthritis [39, 40]. The homocysteine level is significantly increased in OLP patients [41]. Studies have shown that increased MTHFR gene polymorphism is associated with increased levels of homocysteine, dyslipidemia, and increased risk of cardiovascular disease in the OLP patients. This gene is involved in folate metabolism, DNA synthesis, and methylation [42].
The role of vitamin D in regulating immune responses has been approved. Vitamin D inhibits differentiation of B-lymphocytes and secretion of immunoglobulin; also, the proliferation of T-helper cells stimulates regulatory T-cells [43, 44]. Significantly and non-significantly, vitamin D deficiency has been found in OLP patients compared to control (Tables 1 and 5). Although with a higher prevalence of vitamin D deficiency [42, 46, 47], up-regulation of miR-346 and TNF-α and down-regulation of vitamin D receptor were reported to be responsible for the induction of apoptosis in oral mucosal keratinocytes in OLP patients [46]. However, another study showed the down-regulation of miR-802 in relation to decreased vitamin D receptor levels [43].
The level of antioxidants is a potential determinant of susceptibility to OLP. This suggests that oxidative stress is a major trigger for OLP [29].
According to our research we found three categories of studies that evaluated oxidant and antioxidant agents in OLP including, vitamins, carotenoids, and antioxidants and oxidants imbalance.
Vitamin A has critical functions in epithelial differentiation, reducing the rate of epithelial keratinization, anti-inflammatory and immunomodulatory effects, and reproduction [48, 49]. Hairu et al. showed that retinoic acid may suppress excessive T-lymphocyte proliferation and pro-inflammatory cytokines in OLP [50, 51]. According to our research, natural retinol and synthetic analogs both topically and systemically are suggested for OLP treatment (Tables 1 and 4). Compared with corticosteroids, the vitamin A has less therapeutic potency [52], but its combination with steroids has significantly increased therapeutic effects [53], while Cahen et al. showed that the inhibitory effect of vitamin A on lymphocyte function-associated antigen-3 (LFA-3) was greater than that of corticosteroids [54]. Unlike other studies, Nagao reported an increase in retinol levels in the serum of OLP patients though they did not find this as a risk factor for OLP [55].
Vitamins C and E have protective roles against oxidative damage to DNA [29]. Intake of vitamin C from foods may be responsible for a protective effect against head and neck cancers [56]. According to our data, there was a significant decrease in vitamins C and E levels in the OLP patients (Tables 1, 2, and 4). In one study, the levels of these vitamins exhibited a significant increase after curcumin treatment [57]. Administration of selenium with vitamins E and C lead to a significant improvement in OLP lesions compared to corticosteroids [29].
Beta-carotene has a very strong antioxidant and immunoregulatory effect without toxicity [58]. One study demonstrated no significant difference in antioxidant levels in OLP subjects and controls, but lycopene was lower in erosive OLP [55]. Beta-carotene was shown to significantly reduce micronuclei cytoplasmic fragments of DNA in OLP lesions [58]. It increases in response to carcinogenic factors in pre-malignant lesions, indicating recent DNA damage in exfoliated buccal mucosal cells. Therefore, some studies have reported increased levels of some carotenoids in OLP patients (Table 4). Carotenoids enhance the proliferation of B and T-lymphocytes [59].
Oxidant-antioxidant imbalance resulting in excessive accumulation of ROS is defined as oxidative stress [6]. This suggests that patients with OLP are more susceptible to an imbalance of antioxidant-oxidative stress status. Total antioxidant capacity (TAC)/total antioxidant activity (TAA) [60], malondialdehyde (MDA) [61], 8-hydroxy-2¢-deoxyguanosine (8-OHdG) [62], glutathione [6], and uric acid [60] are the potential biomarkers for measuring the effect imbalance of antioxidant-oxidative stress in OLP patients. All of these studies reported a decrease in antioxidant levels and an increase in oxidant levels significantly in OLP patients (Tables 1, 2, 3, and 5). It has been established that OSCC patients are more susceptible to oxidant-antioxidant imbalance than the OLP patients. This may be a valuable finding in the transformation of OLP to OSCC [62].
Given the evidence regarding the deficiency of some micronutrients in OLP patients as well as the satisfactory results of the administration of micronutrients in the recovery process of patients with OLP, it can be suggested that the administration of laboratory tests to the assessment of micronutrients levels are useful to detect deficiency of these substances in such patients.
Conclusion
Based on our review literature, lower levels of iron and its associated markers such as hemoglobin and ferritin, increased level of TIBC, reduced levels of zinc, calcium, vitamin D, vitamin B12, folic acid and antioxidants such as vitamins C and E, and increased levels of oxidants and homocysteine have been reported in OLP patients. Therefore, further clinical and interventional studies are recommended to demonstrate the role of these elements in the pathogenesis and treatment of OLP.
References
Bhattacharya PT, Misra SR (2016) Hussain M: Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica 2016
Wada O: What are trace elements? 2004.
Prashanth L, Kattapagari KK, Chitturi RT, Baddam VRR, Prasad LK (2015) A review on role of essential trace elements in health and disease. J NTR Univ Health Sci 4(2):75
Jolly M, Nobile S (1977) Vitamin status of patients with oral lichen planus. Aust Dent J 22(6):446–450
Gholizadeh N, Sadrzadeh-Afshar M, Mansourian A, Fooladvand S (2018) The relationship between the oral lichen planus and endocrine hormones: a review of literature. Ann Den Spec 6(3):352
Scrobotă I, Mocan T, Cătoi C, Bolfă P, Mureşan A, Băciuţ G (2011) Histopathological aspects and local implications of oxidative stress in patients with oral lichen planus. Rom J Morphol Embryol 52(4):1305–1309
Agha Hosseini F, Sadat Moosavi M, Sadat Sadrzadeh Afshar M, Sheykh Bahaei N (2016) Assessment of the relationship between stress and oral lichen planus: a review of literature. J Islam Dent Assoc Iran 28(2):78–85
Agha-Hosseini F, Sheykhbahaei N, SadrZadeh-Afshar M (2016) Evaluation of potential risk factors that contribute to malignant transformation of oral lichen planus: a literature review. J Contemp Dent Pract 17(8):692–701
Maggini S, Wintergerst ES, Beveridge S, Hornig DH (2007) Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 98(S1):S29–S35
Chen H-M, Wang Y-P, Chang JY-F, Wu Y-C, Cheng S-J, Sun A (2015) Significant association of deficiencies of hemoglobin, iron, folic acid, and vitamin B12 and high homocysteine level with oral lichen planus. J Formos Med Assoc 114(2):124–129
Bao Z-X, Yang X-W, Shi J, Liu L-X (2016) Serum zinc levels in 368 patients with oral mucosal diseases: a preliminary study. Med Oral Patol Oral Cir Bucal 21(3):e335
Kleier C, Werkmeister R, Joos U (1998) Zinc and vitamin A deficiency in diseases of the mouth mucosa. MKG-Chirurg 2(6):320–325
Arora P, Dhillon K, Rajan S, Sayal S, Das A (2002) Serum zinc levels in cutaneous disorders. Med J Armed Forces India 58(4):304–306
Khademi H, Shaikhiany J (2008) Comparisson of serum zing level in recurrent patients and normal individuals. Dent Res J:2(2)
Planus OL: Evaluation of serum zinc level in oral lichen planus, a case control study. 2015.
Ayinampudi BK, Narsimhan M (2012) Salivary copper and zinc levels in oral pre-malignant and malignant lesions. J Oral Maxillofac Pathol JOMFP 16(2):178
Rezazadeh F, Salehi S, Rezaee M (2019) Salivary level of trace element in oral lichen planus, a premalignant condition. Asian Pac J Cancer Prev APJCP 20(7):2009
Mehdipour M, Zenouz AT, Bahramian A, Yazdani J, Pouralibaba F, Sadr K (2010) Comparison of the effect of mouthwashes with and without zinc and fluocinolone on the healing process of erosive oral lichen planus. J Dent Res Dent Clin Dent Prospect 4(1):25
Chaitanya NC, Chintada S, Kandi P, Kanikella S, Kammari A, Waghamare RS (2019) Zinc therapy in treatment of symptomatic oral lichen planus. Indian Dermatol Online J 10(2):174
da Silva PC, de Almeida PV, Machado M, de Lima A, Gregio A, Trevilatto PC, Azevedo-Alanis LR (2008) Oral manifestations of celiac disease. A case report and review of the literature. Med Oral Patol Oral Cir Bucal 13(9):E559–E562
Ma J-M, Wang R, Xu J-Y, Fan Y (2016) Intracellular Ca 2+ and related proteins in patients with oral lichen planus. Immunol Res 64(2):531–539
Wu Y-C, Wang Y-P, Chang JY-F, Cheng S-J, Chen H-M, Sun A (2014) Oral manifestations and blood profile in patients with iron deficiency anemia. J Formos Med Assoc 113(2):83–87
Chang JY-F, Wang Y-P, Wu Y-C, Wu Y-H, Tseng C-H, Sun A (2016) Hematinic deficiencies and anemia statuses in antigastric parietal cell antibody-positive erosive oral lichen planus patients with desquamative gingivitis. J Formos Med Assoc 115(10):860–866
Chang JY-F, Wang Y-P, Wu Y-H, Su Y-X, Tu Y-K, Sun A (2018) Hematinic deficiencies and anemia statuses in anti-gastric parietal cell antibody-positive or all autoantibodies-negative erosive oral lichen planus patients. J Formos Med Assoc 117(3):227–234
Chang JY-F, Chen I-C, Wang Y-P, Wu Y-H, Chen H-M, Sun A (2016) Anemia and hematinic deficiencies in gastric parietal cell antibody-positive and antibody-negative erosive oral lichen planus patients with thyroid antibody positivity. J Formos Med Assoc 115(11):1004–1011
Tiwari R, David CM, Mahesh DR, Sambargi U, Rashmi KJ, Benakanal P (2016) Assessment of serum copper, iron and immune complexes in potentially malignant disorders and oral cancer. Brazil Oral Res 30(1)
Chuykin SV, Akmalova GM, Chuykin OS, Makusheva NV, Akatyeva GG (2016) The role of mineral elements in the pathogenesis of lichen planus of the oral mucosa. Res J Pharm, Biol Chem Sci 7(6):704–710
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268
Belal MH (2015) Management of symptomatic erosive-ulcerative lesions of oral lichen planus in an adult Egyptian population using selenium-ACE combined with topical corticosteroids plus antifungal agent. Contemp Clinical Dent 6(4):454
Wu H, Jiang J, Huang Z, Xiao W, Li Y (2013) Preliminary report of selenium in treatment of oral lichen planus. Stomatology 6:14
SAHEB JM, BEYT EJ, Mansourian A, Shahsavari N, BASIR SS: Assessment of serum vitamin B12 and folic acid in patients with oral lichen planus: a case control study. 2010.
Chang JYF, Chiang CP, Hsiao CK, Sun A (2009) Significantly higher frequencies of presence of serum autoantibodies in Chinese patients with oral lichen planus. J Oral Pathol Med 38(1):48–54
Lin HP, Wang YP, Chia JS, Chiang CP, Sun A (2011) Modulation of serum gastric parietal cell antibody level by levamisole and vitamin B12 in oral lichen planus. Oral Dis 17(1):95–101
Thongprasom K, Youngnak P, Aneksuk V (2001) Folate and vitamin B12 levels in patients with oral lichen planus, stomatitis or glossitis. Southeast Asian J Trop Med Public Health 32(3):643–647
Tulchinsky TH (2010) Micronutrient deficiency conditions: global health issues. Public Health Rev 32(1):243
Nosratzehi T, Ansari MA, Arbabi KF, Maleki L, Amiriyan A: Effect of the serum vitamin B12 and folic acid levels in oral lichen planus. 2013.
Mirzaie A, Hashemi Shahzadeh M, Barzegari M, Azizi A (2018) Comparison of serum folic acid level in oral lichen planus patients and healthy subjects. J Res Dent Maxillofac Sci 3(1):12–15
Lai WKC, Kan MY (2015) Homocysteine-induced endothelial dysfunction. Ann Nutr Metab 67(1):1–12
Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM (1998) Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 55(11):1449–1455
Balkarli A (2016) Relationship between plasma levels of homocysteine and pro-inflammatory cytokines in patients with rheumatoid arthritis. J Clin Exp Investig 7(2):163–167
Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, Girón-Prieto M, Gutiérrez-Salmerón M, García-Mellado V, Cutando A, Naranjo-Sintes R (2011) Lipid levels in patients with lichen planus: a case–control study. J Eur Acad Dermatol Venereol 25(12):1398–1401
Kujundzic B, Zeljic K, Supic G, Magic M, Stanimirovic D, Ilic V, Jovanovic B, Magic Z (2016) Association of vdr, cyp27b1, cyp24a1 and mthfr gene polymorphisms with oral lichen planus risk. Clin Oral Investig 20(4):781–789
Zhao B, Xu N, Li R, Yu F, Zhang F, Yang F, Ge X, Li YC, Du J (2018) Vitamin D/VDR signaling suppresses microRNA-802–induced apoptosis of keratinocytes in oral lichen planus. FASEB J 33(1):1042–1050
Mowry EM, Azevedo CJ, McCulloch CE, Okuda DT, Lincoln RR, Waubant E, Hauser SL, Pelletier D (2018) Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology 91(24):e2256–e2264
Gupta A, Mohan RPS, Kamarthi N, Malik S, Goel S, Gupta S (2017) Serum vitamin D level in oral lichen planus patients of North India-a case-control study. J Dermatol Res Ther 1(2):19
Zhao B, Li R, Yang F, Yu F, Xu N, Zhang F, Ge X, Du J (2018) LPS-induced vitamin D receptor decrease in oral keratinocytes is associated with oral lichen planus. Sci Rep 8(1):763
Du J, Li R, Yu F, Yang F, Wang J, Chen Q, Wang X, Zhao B, Zhang F (2017) Experimental study on 1, 25 (OH) 2D3 amelioration of oral lichen planus through regulating NF-κB signaling pathway. Oral Dis 23(6):770–778
Tanumihardjo SA (2011) Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 94(2):658S–665S
BARUA MDR, RABHA KC, Dutta N (2014) Eefects of vitamin A deficeincy on the cytomorphology of hepatopancreas of Paratelphusa spinigera WOODMASON. Bioscan 9(3):1047–1051
Hairu L, Weiyi Y, Jian Z (1999) Effect of retinoic acid on serum IL-2, sIL-2R, TNF_α levels in patients with oral lichen planus [J]. Label Immunass Clin Med 1
H-r LI, H-o ZHAO, S-j YUAN (2004) Effect of retinoic acid on apoptosis in patients with oral lichen planus [J]. Stomatology 1:8
Buajeeb W, Kraivaphan P, Pobrurksa C (1997) Efficacy of topical retinoic acid compared with topical fluocinolone acetonide in the treatment of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 83(1):21–25
Dalirsani Z, Zenouz AT, Mehdipour M, Alavi F, Javadzadeh Y (2010) Comparison of the effect of combination of triamcinolone acetonide and vitamin A mouthwash with triamcinolone mouthwash alone on oral lichen planus. J Dent Res Dent Clin Dent Prospects 4(1):21
Cahen P, Kirby A, Porter S, Olsen I (2000) Regulation of LFA-3 (CD58) by dexamethasone and retinoic acids in vitro. Inflamm Res 49(7):338–344
Nagao T, Warnakulasuriya S, Ikeda N, Fukano H, Yamamoto S, Yano M, Miyazaki H, Ito Y (2001) Serum antioxidant micronutrient levels in oral lichen planus. J Oral Pathol Med 30(5):264–267
Kaur J, Politis C, Jacobs R (2016) Salivary 8-hydroxy-2-deoxyguanosine, malondialdehyde, vitamin C, and vitamin E in oral pre-cancer and cancer: diagnostic value and free radical mechanism of action. Clin Oral Investig 20(2):315–319
Rai B, Kaur J, Jacobs R, Singh J (2010) Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci 52(2):251–256
Buajeeb W, Kraivaphan P, Amornchat C, Suthamajariya K (2008) Reduction of micronuclei in oral lichen planus supplemented with beta-carotene. J Oral Sci 50(4):461–467
Jones DA (2005) The potential immunomodulatory effects of topical retinoids. Dermatol Online J:11(1)
Miricescu D, Greabu M, Totan A, Didilescu A, Rădulescu R The antioxidant potential of saliva: clinical significance in oral diseases. Molecules 2011, 4(5)
Lopez-Jornet P, Martinez-Canovas A, Pons-Fuster A (2014) Salivary biomarkers of oxidative stress and quality of life in patients with oral lichen planus. Geriatr Gerontol Int 14(3):654–659
Agha-Hosseini F, Mirzaii-Dizgah I, Farmanbar N, Abdollahi M (2012) Oxidative stress status and DNA damage in saliva of human subjects with oral lichen planus and oral squamous cell carcinoma. J Oral Pathol Med 41(10):736–740
Gholizadeh N, Mehdipour M, Najafi S, Bahramian A, Garjani S, Poorfar HK (2014) Evaluation of the serum zinc level in erosive and non-erosive oral lichen planus. J Dent 15(2):52
Mehdipour M, Zenooz AT, Bahramian A, Attaran R, Gholizadeh N, Navard MK (2015) Evaluation of serum calcium level in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol 119(3):e219
Iijima S, Sugiyama Y, Matsumoto N, Kumagai A, Ishibashi S, Sera K (2015) PIXE analysis of trace elements included in oral lichen planus-affected mucosa. Int J PIXE 25(01n02):85–92
Challacombe S (1986) Haematological abnormalities in oral lichen planus, candidiasis, leukoplakia and nonspecific stomatitis. Int J Oral Maxillofac Surg 15(1):72–80
Patel S, Yeoman C, Murphy R (2005) Oral lichen planus in childhood: a report of three cases. Int J Paediatr Dent 15(2):118–122
Rashed L, Abdel Hay R, AlKaffas M, Ali S, Kadry D, Abdallah S (2017) Studying the association between methylenetetrahydrofolate reductase (MTHFR) 677 gene polymorphism, cardiovascular risk and lichen planus. J Oral Pathol Med 46(10):1023–1029
Bahramian A, Bahramian M, Mehdipour M, Falsafi P, Khodadadi S, Tabriz FD, Deljavanghodrati M (2018) Comparing vitamin D serum levels in patients with oral lichen planus and healthy subjects. J Dent 19(3):212
Ergun S, Troşala ŞC, Warnakulasuriya S, Özel S, Önal AE, Ofluoğlu D, Güven Y, Tanyeri H (2011) Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. J Oral Pathol Med 40(4):286–293
Nicolae I, Mitran CI, Mitran MI, Ene CD, Tampa M, Georgescu SR (2017) Ascorbic acid deficiency in patients with lichen planus. J Immunoass Immunochem 38(4):430–437
Helms AE, Brodell RT (2002) Scurvy in a patient with presumptive oral lichen planus. Nutr Clin Pract 17(4):237–239
Abdolsamadi H, Rafieian N, Goodarzi MT, Feradmal J, Davoodi P, Jazayeri M, Taghavi Z, Hoseyni S-M, Ahmadi-Motamayel F (2014) Levels of salivary antioxidant vitamins and lipid peroxidation in patients with oral lichen planus and healthy individuals. Chonnam Med J 50(2):58–62
Petruzzi M, De Benedittis M, Grassi R, Cassano N, Vena G, Serpico R (2002) Oral lichen planus: a preliminary clinical study on treatment with tazarotene. Oral Dis 8(6):291–295
Kunz M, Urosevic-Maiwald M, Goldinger S, Frauchiger A, Dreier J, Belloni B, Mangana J, Jenni D, Dippel M, Cozzio A (2016) Efficacy and safety of oral alitretinoin in severe oral lichen planus–results of a prospective pilot study. J Eur Acad Dermatol Venereol 30(2):293–298
Kolios AG, Maggio EM, Gubler C, Cozzio A, Dummer R, French LE, Navarini AA (2013) Oral, esophageal and cutaneous lichen ruber planus controlled with alitretinoin: case report and review of the literature. Dermatology 226(4):302–310
Boisnic S, Licu D, Ben LS, Branchet-Gumila M, Szpirglas H, Dupuy P (2002) Topical retinaldehyde treatment in oral lichen planus and leukoplakia. Int J Tissue React 24(4):123–130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gholizadeh, N., Sheykhbahaei, N. Micronutrients Profile in Oral Lichen Planus: a Review Literature. Biol Trace Elem Res 199, 912–924 (2021). https://doi.org/10.1007/s12011-020-02221-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02221-9