Abstract
Nanoparticle-enhanced coatings of bone implants are a promising method to facilitate sustainable wound healing, leading to an increase in patient well-being. This article describes the in vitro characterization of osteoblast cells interacting with polyelectrolyte multilayers, which contain detonation nanodiamonds (NDs), as a novel class of carbon-based coating material, which presents a unique combination of photoluminescence and drug-binding properties. The cationic polyelectrolyte, namely polydiallyldimethylammonium chloride (PDDA), has been used to immobilize NDs on silica glass. The height of ND-PDDA multilayers varies from a minimum of 10 nm for one bilayer to a maximum of 90 nm for five bilayers of NDs and PDDA. Human fetal osteoblasts (hFOBs) cultured on ND-PDDA multilayers show a large number of focal adhesions, which were studied via quantitative fluorescence imaging analysis. The influence of the surface roughness on the filopodia formation was assessed via scanning electron microscopy and atomic force microscopy. The nano-rough surface of five bilayers constrained the filopodia formation. The hFOBs grown on NDs tend to show not only a similar cell morphology compared to cells cultured on extracellular matrix protein-coated silica glass substrates, but also increased cell viability by about 40%. The high biocompatibility of the ND-PDDA multilayers, indicated via high cell proliferation and sound cell adhesion, shows their potential for biomedical applications such as drug-eluting coatings and biomaterials in general.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, localized drug delivery applications via nanocarriers have gained the attention of the scientific community [1–3]. Such nanocarriers are known to optimize therapeutic indices and to increase the bioavailability of tethered active pharmaceutical ingredients [4, 5]. Different nanocarriers consisting of liposomes, micelles, nanoemulsions, virus-based, inorganic, magnetic, and polymeric nanoparticles are widely discussed [6].
Within this spectrum, detonation nanodiamonds (NDs) are favorable to tether diverse biomolecules and to facilitate the study of drug delivery processes. NDs discovered in the 1960s, possess a unique combination of properties including a high diversity of functional surface groups, intrinsic photoluminescence, and superior mechanical characteristics [7]. Thus, NDs show tremendous potential in the field of biomedical research with applications in oncology, imaging techniques, tissue implants, antimicrobial agents, and drug delivery systems [8, 9]. Nanocarriers based on NDs have been investigated in the context of anticancer therapy and gene delivery. Zhang et al presented the multimodal functionalization of NDs with chemotherapeutics and specific antibodies to promote the internalization and enhanced therapeutic activity of the ND conjugates in solution [10]. In order to create highly effective in vivo anticancer nucleic-acid nanocarriers, NDs were functionalized with small interfering RNA, which shows low toxicity compared to common transfection agent [11]. Further biomolecules such as growth factors and anti-inflammatory drugs have also been grafted onto NDs. Octadecylamine and dexamethasone functionalized NDs are such examples which exhibit strong anti-inflammatory and pro-phagocytic effects on macrophage gene expression [12]. Moreover, monolayers of physisorbed NDs promote the formation of functional neuronal networks, bypassing the necessity of protein coatings [13]. NDs have been applied as a coating material for tissue engineering applications due to their functionalization capabilities. Polymeric scaffolds have been modified by physisorption of bone morphogenetic protein-2 functionalized NDs to facilitate the bone formation [14].
In particular, the delivery of therapeutics from the implant site to bones to treat skeletal diseases or to prevent post-surgical infections is challenging due to the complex and solid bone structure that limits blood supply and diffusion of therapeutics administered by systemic routes to reach an effective concentration [15]. Therefore, the local drug delivery to bone has been recognized to overcome the limitations of the conventionally utilized therapies [16, 17]. Accordingly, drug-release coatings that combine common coating technologies with nanocarriers are a promising basis for a myriad of applications [18–20]. The release of drugs and nanocarriers from coatings can be controlled by (1) degradation of the coating, (2) diffusion due to a porous structure of the coating, or (3) solution flow based on dedicated drug reservoirs from the surface of the coating [21]. Polyelectrolyte multilayers (PEMs) are able to address both diffusion and degradation controlled release of drugs and nanocarriers based on the appliedpolyelectrolyte [22, 23]. PEMs consist of alternately charged polyanionic and polycationic layers [24]. Moreover, PEMs are already recognized as coating materials for nanoparticles in drug delivery applications [25].
The present study describes the novel design and the in vitro characterization of ND-polydiallyldimethylammonium chloride (PDDA) multilayers for bone implant applications. The method to immobilize various amounts of NDs on macroscopic surfaces via PEMs and the means to control the resulting surface properties are discussed. The cell-material interactions of human fetal osteoblasts (hFOBs) cultured on ND-PDDA multilayers have been investigated, where the hFOBs exhibited a high cell adhesion and high cell viability. The high biocompatibility of ND-PDDA multilayers indicates their tremendous potential in biomedical applications. Hence, the study discusses the initial step to advance the research on NDs as nanocarriers for biomaterial coatings and to tailor the number of nanocarriers in the coating with respect to the course of the target disease.
2. Methods
2.1. Materials and reagents
Detonation NDs were purchased from PlasmaChem GmbH, Berlin, Germany. The declared average particle size is 4 nm. Silica glass slides were obtained from Corning Korea Company Ltd., Seoul, Republic of Korea. Ultrapure water (ddH2O) was collected using a DI-water generator (EXL-3 Water Purification System by Vivagen Co., Ltd., Seongnam, Republic of Korea). PDDA solution (20 wt% in H2O, 200.000 < Mw < 350.000), and all other reagents used in this work were purchased from Merck KGaA, Darmstadt, Germany and used without further purification.
2.2. De-agglomeration of NDs and application of PEMs
NDs were suspended in ddH2O to a final concentration of 1 mg ml−1 and de-agglomerated using the salt-assisted ultrasonic de-aggregation as described elsewhere [26]. The absence of chloride in the obtained ND suspension was proved using Mohr's method.
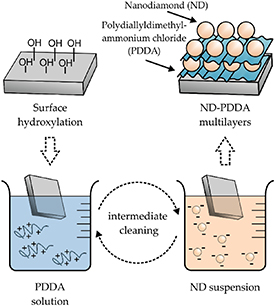
Silica glass substrates were consecutively cleaned using 5 min ultrasound treatment (Branson 5510 R-DTH by Emerson Electric Company, St. Louis, MO, USA) in acetone, isopropanol, and ddH2O. Subsequently, the silica glass substrates were treated for 60 sec in O2 plasma with an operating pressure of 400 mTorr, an oxygen flow rate of 40 sccm, and a plasma power of 200 W using an O2 plasma asher by SNTEK Co., Ltd, Suwon-si, Republic of Korea. ND-PDDA multilayers were obtained via layer-by-layer processing as schematically illustrated in figure 1. The cleaned and O2 plasma-treated silica glass substrates were successively dip-coated for 10 min in 50 ml of PDDA solution (cPDDA = 2 mg ml−1) and 50 ml of de-agglomerated ND suspension (cND = 1 mg ml−1) with an intermediated rinsing step of 10 min in ddH2O. The maximum of five bilayers of NDs and PDDA was applied, where the final layer always consisted of NDs.
Figure 1. Schematic coating method of detonation NDs using PEMs.
Download figure:
Standard image High-resolution image2.3. Cell culture and imaging
All substrates were washed with 70% ethanol and placed in 12-well cell culture plates. In order to facilitate cell attachment on the control, bare silica glass substrates were coated with fibronectin (FN) (Sigma-Aldrich Co., St. Louis, MO, USA) before cell seeding. In brief, bare silica glass substrates was soaked into 30 ng µl−1 FN in phosphate-buffered saline (PBS) for 4 h and dried at room temperature (RT) under room air conditions for 1 h. hFOBs (hFOB 1.19; ATCC® CRL-11 372™, Manassas, VA, USA) were cultivated in Dulbecco's Modified Eagle Medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) at 5% CO2 and 37 °C for 24 h. The cell suspension of 5 × 104 cells was resuspended and seeded on the substrates in the cell culture plates. After several hours of incubation, the cell culture medium was changed to maintain only adhered cells. After 24 h of cultivation, substrates were washed with PBS and the adhered cells were used to conduct immunofluorescence staining. Cells were fixed using 4% paraformaldehyde in PBS at RT for 20 min. Before staining, the fixed cells were subsequently permeabilized with 0.5% Triton X-100 (permeabilization agent, Sigma) for 5 min and blocked in 5% bovine serum albumin (BSA, Millipore, Burlington, MA, USA) in PBS at RT for 60 min. The samples were incubated with a mixture of vinculin primary antibody (1:200, Sigma) diluted in 5% in BSA and Alexa Fluor™ 633 Phalloidin diluted in BSA (1:40, Thermo Fisher Scientific) at 37 °C for 1 h. The substrates were washed two times with PBS to remove any excess, followed by incubation with a fluorescence-labeled secondary antibody (goat anti-mouse Alexa Fluor™ 488, Thermo Fisher Scientific) diluted in 5% in BSA at RT for 30 min. Finally, cell nuclei were stained by mounting solution with DAPI (4',6-diamidino-2-phenylindole) from Vector Lab Inc. Burlingame, CA, USA.
The fluorescence images of the stained hFOBs were obtained using confocal microscopy with ZEN software (LSM700, Carl Zeiss, Oberkochen, Germany). Fluorescence images were analyzed using ImageJ software (National Institutes of Health, USA) [27]. Protein distribution per cell and area of cells as a degree of spreading were expressed as mean ± standard deviation (SD) and studied via t-test to determine statistical relevance.
The surface morphology and roughness were analyzed using scanning electron microscopy (SEM) (JSM-7100 F, JEOL, Tokyo, Japan) and atomic force microscopy (AFM) (Park Systems, XE100, Seoul, Republic of Korea), respectively.
2.4. Cell viability
Cell viability was measured using colorimetric MTT cell proliferation assay. The hFOBs were cultured in 12-well cell culture plates, with six duplicate wells in each group. The cells were incubated overnight at 37 °C. Then, the cell culture medium was removed and washed with PBS. The cells were cultured for 3 h after 500 µl of MTT reagent was dispensed. When the purple precipitate was observed using optical microscopy, 200 µl of dimethyl sulfoxide (DMSO, Sigma) was carefully added and the obtained mixture was transferred to an ELISA plate. The absorbance in each well was measured using the microplate reader VersaMax™ by Molecular Devices, San Jose, CA, USA with a 570 nm-wavelength filter. The mean optical density (OD, absorbance) of six wells in the indicated groups was used to calculate the percentage of cell viability as follows: percentage of cell viability = (ODsample−ODblank)/(ODcontrol−ODblank) × 100%. The error propagation was calculated in regard to the maximum uncertainty.
3. Results
3.1. Topography of ND-PDDA multilayers on silica glass
Multiple bilayers of detonation NDs and PDDA have been applied on silica glass to tailor the amount of NDs on the surface and to regulate the surface roughness. Figure 2 exhibits the SEM and AFM images of ND-PDDA multilayers as well as the correlated surface roughness and surface area. The positively charged polyelectrolyte PDDA is able to homogenously immobilize the negatively charged NDs on silica glass. Accordingly, figures 2(a) and (d) describe the bare silica glass surface without ND-PDDA multilayers. Figures 2(b) and (e) show one bilayer while figures 2(c) and (f) illustrate five bilayers of NDs and PDDA. The immobilized NDs cover the entire surface in the case of the employed one and five bilayers. The height of one bilayer ranges from 10–50 nm deduced from AFM measurements. All further applied bilayers are going to coarsen the roughness based on the initial ND agglomerate size distribution. Five bilayers of NDs and PDDA form the roughest surface with a maximum height of 90 nm, and the immobilized NDs are packed closer to each other compared to one bilayer. Figure 2(g) displays the correlated surface roughness values Rq for the respective surface modification. Five bilayers of NDs and PDDA provide the highest roughness value of 43 ± 4 nm, whereas one bilayer shows a lower roughness values of 25 ± 5 nm. The lowest roughness was obtained from silica glass with FN coating possessing an Rq of 1.3 ± 0.1 nm. Figure 2(h) shows the increase in the surface area along with the iteration of ND-PDDA bilayers. The geometrical reference area was 10 µm × 10 µm. The surface areas of one bilayer and five bilayers are increased by 2.5% and 5.0% compared to silica glass with FN coating.
Figure 2. (a)–(c) SEM and (d)–(f) AFM images of ND-PDDA multilayers on silica glass. (a) +(d) Bare silica glass, (b) +(e) one bilayer and (c) +(f) five bilayers of NDs and PDDA. (g) Surface roughness Rq in nm and (h) correlated surface area of ND-PDDA multilayers; reference area for measurement 100 μm2.
Download figure:
Standard image High-resolution image3.2. Cell morphology
Cell morphological changes provide important information about cell differentiation processes, cell functions, and signal responses [28]. The morphology of adherent hFOBs has been assessed by the extent of the cell spreading obtained via fluorescence microscopy and SEM. Figure 3 displays the calculated mean area of the adherent cells and the correlated merged fluorescent microscopy images. The mean cell area, the shape of the cytoskeleton, and the distribution of cells, which were cultured on ND-PDDA multilayers and silica glass with FN coating, are comparable. As shown in figure 3(a), the difference in the mean area of adherent cells on one bilayer and five bilayers of NDs and PDDA is non-significant compared to silica glass with FN coating. The mean area of adherent cells on the silica glass with FN coating is 2040 ± 800 μm2, one bilayer 1460 ± 630 μm2, and five bilayers 1060 ± 650 μm2. This outcome correlates with the representative fluorescence microscopy images displayed in figures 3(b)–(d). The hFOBs cultured on ND-PDDA multilayers and silica glass with FN coating demonstrate a comparable shape and spreading behavior using fluorescence microscopy. In contrast, figure 3(e) depicts the hFOBs cultured on a monolayer of PDDA, which exhibit a discrepancy in the mean cell area and cell shape compared to cells cultured on ND-PDDA multilayers and silica glass with FN coating. The complex actin network and focal adhesions, which are associated with the vinculin content, are indiscrete and coinciding in the images [29]. The hFOBs cultured on a monolayer of PDDA try to minimize their surface, which results in a minimal mean area of the adherent cells of 520 ± 30 μm2.
Figure 3. (a) Mean area of adherent hFOBs on the respective surface modification. Unpaired t-test (n = 3) *P > 0.05; **P < 0.01. Merged fluorescence microscopy images of hFOBs on (b) silica glass (with FN coating), (c) one bilayer, (d) five bilayers of NDs and PDDA, and (e) a monolayer of PDDA. Scale 100 μm; red—F-actin, blue—cell nuclei, green—vinculin.
Download figure:
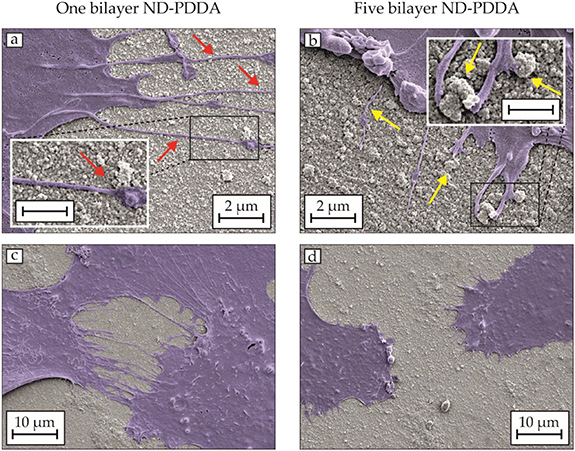
Standard image High-resolution imageIn order to compare the morphological changes of hFOBs on ND-PDDA multilayers, SEM imaging has been conducted. Figure 4 exhibits the correlated SEM images of the filopodia formation and the differences of the adherent hFOBs along the nanoroughness that is rather randomly distributed. Filopodia are highly dynamic cell-surface protrusions used by cells to sense their external environment [30]. The hFOBs on one bilayer exhibit a larger number of thicker filopodia, which grew in a straighter direction, whereas those on five bilayers avoid large ND agglomerates, as highlighted in figures 4(a) and (b). The red arrows indicate the filopodia, which were able to pass over the ND agglomerates of one bilayer, and the yellow arrows highlight that the filopodia were either blocked or avoided by the large ND agglomerates of five bilayers. The increase in the nanoroughness inhibits the filopodia formation and disturb the communication between cells living close to each other. Figures 4(c) and (d) displays the overview of cultured hFOBs, where the cells on the less rough surfaces of one bilayer form a higher number of defined filopodia compared to five bilayers of NDs and PDDA.
Figure 4. SEM images of the hFOB filopodia grown on (a) +(c) one bilayer and (b) +(d) five bilayers of NDs and PDDA for 24 h. Scale of insets 1 µm.
Download figure:
Standard image High-resolution image3.3. Cell adhesion
Figure 5 illustrates the mean intensity of vinculin per cell on ND-PDDA multilayers. The mean vinculin intensity of hFOBs cultured on one bilayer and five bilayers of NDs and PDDA are slightly increased compared to silica glass with FN coating, possessing total values of 104 ± 20% and 107 ± 16%, respectively. The difference in the mean vinculin intensity is non-significant. On the contrary, the mean vinculin intensity decreases to a total value of 74 ± 17% in the case of silica glass without FN coating, which represents a significantly lower amount of vinculin compared to the ND-PDDA multilayers. This outcome confirms that ND-PDDA multilayers promote the adhesion of hFOBs to a similar extent as substrates with FN coatings.
Figure 5. Mean intensity of vinculin per cell in percent of the control for one bilayer and five bilayers of NDs and PDDA after 24 h of incubation. Welch's unpaired t-test (n > 30) *P > 0.05; **P < 0.01.
Download figure:
Standard image High-resolution image3.4. Cell viability
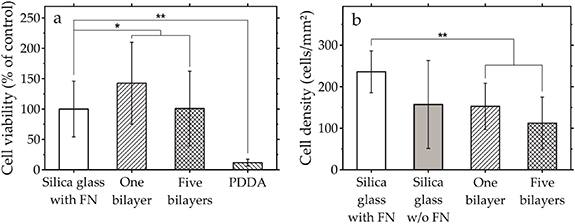
The biocompatibility of the ND-PDDA multilayers has been assessed using the metabolic cell activity and cell density of hFOBs. The determination of the cell viability is broadly used to evaluate the in vitro biocompatibility of various materials. Figure 6 illustrates the cell viability of hFOBs and the cell density of hFOBs. Figure 6(a) shows the cell viability of hFOBs cultured on ND-PDDA multilayers and on a monolayer of PDDA after 24 h of incubation. Once the hFOBs are exposed to the ND-PDDA multilayers, the cell viability increases to a total value of 143 ± 67% for one bilayer and 101 ± 61% for five bilayers in comparison to silica glass with FN coating. The difference in cell viability between the populations of ND-PDDA multilayers and silica glass with FN coating are non-significant. Accordingly, the ND-PDDA multilayers possess the same biocompatibility as FN-coated substrates. Since the applied multilayers consist of NDs and PDDA, the influence of PDDA on the viability of hFOBs has been investigated. PDDA leads to a significantly lower cell viability with 12 ± 6% of living hFOBs compared to silica glass with FN coating. Figure 6(b) shows the cell density of hFOBs on each substrate after 24 h of incubation using optical microscopy image analysis. The number of adhered hFOBs on ND-PDDA multilayers decreases compared to the number of cells on silica glass with FN coating.
Figure 6. (a) Cell viability in percent of hFOBs cultured on ND-PDDA multilayers and on a monolayer of PDDA and (b) cell density of adherent hFOBs after 24 h of incubation. Unpaired t-test (n = 6) *P > 0.05; **P < 0.01.
Download figure:
Standard image High-resolution image4. Discussion
The present study evaluates the cellular response of hFOBs to ND-PDDA multilayers and discusses an application-relevant coating technique. NDs have been immobilized on silica glass using the self-assembly process of PEMs. Therefore, alternate charged layers of polyelectrolytes and NDs have been deposited leading to coatings applicable for numerous materials. The height of the applied ND-PDDA multilayers varies from 10 nm to 90 nm due to the initial ND agglomerate size distribution and the applied quantity of bilayers.
No linear correlation between the acquired ND-PDDA multilayer heights and the number of applied bilayers was detected. This outcome can be explained following the space-filling mechanism of PEMs containing nanoparticles [31]. Small ND agglomerates penetrate the existing bilayer and occupy the interspaces of the prevailing ND structures. Hence, the prevalent bilayer of NDs and PDDA influences the morphology of the following bilayer. The understanding of the ND coating behavior using PEMs is the key to predict the resulting yield of NDs on macroscopic surfaces and to promote such systems in the direction of drug-eluting coatings. The interactions between the substrate, ND agglomerates, and polyelectrolytes are based on electrostatic attractions [32]. Furthermore, hydrogen bonding facilitates the formation of PEMs [33]. The applied polyelectrolyte PDDA belongs to the group of polycations and is able to interact with the negatively charged ND agglomerates. PDDA is widely used to modify nanoparticulate carriers and to immobilize nanoparticles on macroscopic surfaces [34, 35]. The employed salt-assisted ultrasonic deaggregation (SAUD) technique was not capable to disaggregate the ND agglomerates. The observed ND agglomerate size was larger compared to the values reporter in the literature, due to a slightly modified protocol and lower concentrations of NDs utilized during the disaggregation technique [26]. NDs principally agglomerate due to contaminations by heavy metals, Coulombic inter-particle attractions, and covalent bonds [36–38]. The absence of chloride in the ND suspensions has been proved using Mohr's method and further aggregation due to interactions of NDs with NaCl ions can be excluded.
Dierich et al characterized PEMs utilizing growth factors and showed the osteogenic differentiation of stem cells induced from the released biomolecules [39]. However, NDs as drug delivery vehicles have the possibility for a more controlled in vivo release of the biomolecules. NDs have been investigated previously as delivery platforms for growth factors to promote bone formation [40]. Hence, ND-PDDA multilayers have been applied in the present work to control the density and yield of immobilized NDs on macroscopic surfaces and to combine the potential of PEMs and NDs for future drug-release studies.
The morphology of adherent hFOBs was assessed by the extent of the cell spreading obtained via fluorescence microscopy. The mean area of adherent hFOBs tends to decrease on five bilayers (rougher surface, Rq > 40 nm) compared to one bilayer (smoother surface, Rq < 25 nm). The reduced spreading of the cells can be explained by comparing the filopodia formation on the ND-PDDA multilayers possessing different surface roughnesses. Filopodia play an important role in sensing surface topography and are associated with cell spreading and migration [41, 42]. The growth and extension of filopodia are reduced on five bilayers of NDs and PDDA with a high surface roughness, which can be understood as the relative decrease in cell spreading and migration. On the other hand, filopodia can grow on one bilayer and are not affected by the surface features due to the smoother surface. The degree of filopodia formation appears to be highly dependent on the height of the ND agglomerates. This outcome agrees with previous reports that filopodia formation was extensively limited in regions with higher roughnesses [43]. Biggs et al described that a 70–100 nm height of protrusion reduced the cell adhesion and the focal adhesion complex formed again when the height was under 50 nm [44]. However, cells cultured on ND-PDDA multilayers exhibit a similar cell shape and cytoskeletal organization compared to silica glass with FN coating, suggesting that cells possess undisturbed signal transduction pathways and ultimately comparable cell health [45].
The cell adhesion is the initial phase for vital cell activity and conclusively influences the development of the tissue [46]. In order to compare cell adhesion on different surfaces, the expression level of vinculin was measured by quantitative fluorescence imaging analysis [47]. Vinculin is a protein that constitutes the integrin-mediated focal adhesion and can be compared to assess the direct cell contact [48]. Moreover, the formation of the cell adhesion complex via vinculin is related to a biochemical attachment of hFOBs, which furthermore suggests that the substrate possesses high biocompatibility [49].
The expressed amount of vinculin has been used to assess the cell adhesion. The hFOBs cultured on ND-PDDA multilayers exhibit a similar amount of vinculin compared to cells cultured on silica glass with FN coating. In contrast, hFOBs on bare silica glass comprises a significantly lower amount of vinculin compared to ND-PDDA multilayers. The outcome suggests that NDs promote the formation of focal adhesion to the same extent as FN coatings. FN is part of the extracellular matrix and contains tripeptide arginine-glycine-aspartic acid, a motif mediating cell adhesion [50, 51]. It should be noted that the surface area of ND-coated substrates increases with the number of the applied bilayer. However, no influence of the resulting surface area was found on the vinculin level.
The ND-PDDA multilayers exhibit high biocompatibility. The cell viability of hFOBs cultured on ND-PDDA multilayers was over 100% in comparison to silica glass with FN coating. Moreover, the hFOBs showed non-significant higher cell viability in the case of one bilayer compared to five bilayers of NDs and PDDA, which correlates with the observed filopodia formation and the surface roughness of the substrates. In general, substrates with a high nanoroughness lead to reduced proliferation of osteo- and fibroblasts [52, 53]. Furthermore, the higher amount of PDDA in the case of five bilayers may result in lower cell viability due to the dose-dependent cytotoxic character of the polyelectrolyte [54]. Accordingly, the hFOBs cultured on a monolayer of PDDA try to minimize their surface, leading to significantly smaller mean cell areas, the smallest number of adherent cells, and low cell viability. The cell behavior on various surface modifications strongly depends on several factors such as surface chemistry, surface feature sizes, cultivation period of the cells, and cell lineage [55,56–58]. However, Xu et al showed the higher cell adhesion of fibroblasts on positively charged surfaces compared to their negatively charged counterparts and discussed the influence of the underlying topography [59]. The present outcome suggests that negatively charged and nanostructured surfaces are favorable for hFOBs compared to positively charged and smooth surfaces. At the same time, the highest number of adherent hFOBs was observed for silica glass with FN coating due to its high affinity for cell adhesion. Despite the high cell viability of hFOBs cultured on ND-PDDA multilayers, they exhibit the smallest number of adherent cells. In order to improve the significance of the obtained results, the performance of long-term proliferation assays is proposed [60].
Further investigations related to the translational relevance and the release kinetics of drug-conjugated NDs have to be addressed concerning their specific application. Potential release mechanisms of the NDs from PEMs are the photocatalytic decomposition, application of biodegradable polyelectrolytes, or dissociation based on the local alteration of physiological salt concentration and pH in human body fluids [61–63]. The advantages of the ND-PDDA multilayers are the high flexibility of the employed PEMs regarding the substrate material and the possibility to create multifunctional surfaces. The disadvantages are the currently applied polyelectrolyte, namely PDDA, and the insufficient understanding of the influence of the surface charge on the cellular response [64]. However, a monolayer of negatively charged NDs cancels the cytotoxic effect of the positively charged polyelectrolyte PDDA. Consequently, ND-PDDA multilayers are considered as biocompatible material suitable for biomedical applications, especially for bone implants.
5. Conclusion
The in vitro characterization of ND-PDDA multilayers is the key to address the translational relevance of NDs and to predict their efficiency in clinical trials. The cell morphology, cell adhesion, and cell viability of hFOBs cultured on ND-PDDA multilayers have been investigated for bone implant applications. The ND-PDDA multilayers possess high biocompatibility assessed using the analysis of the hFOB viability. Additionally, the hFOBs adhere to ND-PDDA multilayers to the same extent as on extracellular matrix protein-coated substrates. Despite the high vinculin content of hFOBs seeded on ND-PDDA multilayers, the adherent cells exhibit a similar mean area and a smaller number compared to cells cultured on silica glass with an extracellular matrix protein coating. This behavior is related to the nanoroughness of the substrates, which accordingly influences the filopodia formation of hFOBs. The outcome proves the feasibility of NDs as a coating material for biomedical applications and as promising drug delivery vehicles.
Acknowledgments
We gratefully acknowledge the support through the International Excellence Graduate School on Emerging Materials and Processes Korea (iEGSEMP Korea) in the context of TU Dresden's Institutional Strategy (ZUK 64), funded by the Excellence Initiative of the German Federal and State Governments. This research was supported by the MSIT (Ministry of Science and ICT), Korea, under the ICT Consilience Creative program (IITP-2019-2017-0-01015) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation) and by the Basic Science Research Program (NRF-2018R1D1A1B07042537) supervised by the National Research Foundation of Korea (NRF) funded by the Ministry of Education in Republic of Korea. Sascha Balakin and Young-Shik Yun contributed equally to this work.