Abstract
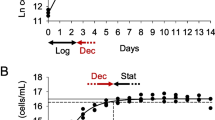
This study assessed in vitro interaction between Bacillus bacteria and microalgae and their posterior in vivo effect on rearing Kumamoto oyster Crassostrea sikamea. The probiotic strains Bacillus licheniformis (MAt32), B. subtilis (MAt43) and B. subtilis (GAtB1) were individually inoculated in triplicate into 250 mL flasks containing 1 × 104 colony forming units (CFU) mL−1 of bacteria and 4.5 × 104 cell mL−1 of microalgae (Isochrysis galbana or Chaetoceros calcitrans) to evaluate their growth during a 7-day culture. Single cultures of microalgae or bacilli served as control. Additionally, C. sikamea spat was treated for 28 days with four single/combined bacillus treatments in triplicate at a concentration of 1 × 106 CFU mL−1 as follows: (a) control, without treatments; (b) combination of two antibiotics (10 mg L−1); (c) B. licheniformis; (d) B. subtilis; (e) B. subtilis subtilis and (f) mixed bacilli. The results showed a significantly (P < 0.05) increased growth of Bacillus strains co-cultured with microalgae, while the growth of I. galbana co-cultured with bacteria was not reduced significantly (P > 0.05) compared with the control group. C. sikamea spat treated with Bacillus showed significantly (P < 0.05) higher growth and survival than the control group. In this study, C. calcitrans microalgae were susceptible to the presence of probiotic bacteria. Nonetheless, this reduction in microalgal growth observed in vitro increased growth and survival of C. sikamea spat exposed to probiotic bacteria when compared to spat without probiotics.



Similar content being viewed by others
References
Camara MD, Davis JP, Sekino M, Hedgecock D, Li G, Langdon CJ, Evans S (2008) The Kumamoto oyster Crassostrea sikamea is neither rare nor threatened by hybridization in the northern Ariake sea, Japan. J Shellfish Res 27:313–322
Wang H, Qian L, Wang A, Guo X (2013) Occurrence and Distribution of Crassostrea sikamea (Amemiya 1928) in China. J Shellfish Res 32:439–446. https://doi.org/10.2983/035.032.0224
Sekino M (2009) In search of the Kumamoto oyster Crassostrea sikamea (Amemiya, 1928) based on molecular markers: is the natural resource at stake? Fish Sci 75:819–831. https://doi.org/10.1007/s12562-009-0100-6
Cáceres-Martínez J, Vásquez-Yeomans R, Guerrero-Rentería Y (2012) Early gametogenesis of Kumamoto oyster (Crassostrea sikamea). Hidrobiológica 22(2):181–184
Elston RA, Moore J, Abbott CL (2012) Denman Island disease (causative agent Mikrocytos mackini) in a new host, Kumamoto oysters Crassostrea sikamea. Dis Aquat Organ 102:65–71. https://doi.org/10.3354/dao02519
Cáceres-Martínez J, Vásquez-Yeomans R (2013) Enfermedades, parásitos y episodios de mortalidad de ostiones de importancia comercial en México y sus implicaciones para la producción. Cienc Pesq 21:5–48
Trabal-Fernández N, Mazón-Suástegui JM, Vázquez-Juárez R, Ascencio-valle F, Romero J (2014) Changes in the composition and diversity of the bacterial microbiota associated with oysters (Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea) during commercial production. FEMS Microbiol Ecol 88:69–83. https://doi.org/10.1111/1574-6941.12270
Sainz J, Maeda-Martínez A, Ascencio F (1998) Microb Ecol 35:188. https://doi.org/10.1007/s002489900073
Campa-Córdova AI, Luna-González A, Zarain-Herzberg M, Cáceres-Martínez JC (2005) Prophylactic use of antibiotics in larval culture of Argopecten ventricosus (Sowerby, 1835). J Shellfish Res 24(4):923–930. https://doi.org/10.2983/0730-8000
Li N, Lin Q, Fu X, Guo H, Liu L, Wu S (2015) Development and efficacy of a novel streptomycin-resistant Flavobacterium johnsoniae vaccine in grass carp (Ctenopharyngodon idella). Aquaculture 448:93–97. https://doi.org/10.1016/j.aquaculture
Holmström K, Gräslund S, Wahlströom A, Poungshompoo S, Bengtsson BE, Kautsky N (2003) Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int J Food Sci Technol 38:255–266. https://doi.org/10.1046/j.1365-2621.2003.00671.x
Nwachi OF (2013) An overview of the importance of probiotics in aquaculture. J Fish Aquat Sci 8:30–32
Navarrete P, Caruffo M (2015) Antibiotics in aquaculture: impacts and alternatives. APUA Newsl 33(2):4–7
Martínez-Córdova LR, Emerenciano M, Miranda-Baeza A, Martínez-Porchas M (2015) Microbial-based systems for aquaculture of fish and shrimp: An updated review. Rev Aquac 7:131–148. https://doi.org/10.1111/raq.12058
Zhang Q, Tan B, Mai K, Zhang W, Ma H, Ai Q, Wang X, Liufu Z (2011) Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora, immunological parameters and resistance against Vibrio alginolyticus in shrimp, Penaeus japonicus (Decapoda: Penaeidae). Aquac Res 42:943–952. https://doi.org/10.1111/j.1365-2109.2010.02677.x
Tuan TN, Duc PM, Hatai K (2013) Overview of the use of probiotics in aquaculture. Int J Res Fish Aquac 3(3):89–97
Abasolo-Pacheco F, Saucedo PE, Mazón-Suástegui JM, Tovar-Ramírez D, Araya R, Ramírez-Orozco JM, Campa-Córdova ÁI (2016) Isolation and use of beneficial microbiota from the digestive tract of lions-paw scallop Nodipecten subnodosus and winged pearl oyster Pteria sterna in oyster aquaculture. Aquac Res 47:3042–3051. https://doi.org/10.1111/are.12754
Escamilla-Montes R, Luna-González A, Flores-Miranda MC, Álvarez-Ruiz P, Fierro-Coronado JA, Sanchez-Ortiz AC (2015) Isolation and characterization of potential probiotic bacteria suitable for mollusk larvae cultures. Thai J Veterenary Med 45(1):11–21
Prado S, Romalde JL, Barja JL (2010) Review of probiotics for use in bivalve hatcheries. Vet Microbiol 145:187–197. https://doi.org/10.1016/j.vetmic.2010.08.021
Gibson LF, Woodworth J, George AM (1998) Probiotic activity of Aeromonas media on the Pacific oyster, Crassostrea gigas, when challenged with Vibrio tubiashii. Aquaculture 169:111–120
Fuentes J, Garbayo I, Cuaresma M, Montero Z, González-del-Valle M, Vílchez C (2016) Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar Drugs 14:100. https://doi.org/10.3390/md14050100
Avendaño RE, Riquelme CE (1999) Establishment of mixed-culture probiotics and microalgae as food for bivalve larvae. Aquac Res 30:893–900
Sánchez-Ortiz AC, Luna-González A, Campa-Córdova ÁI, Escamilla-Montes R, Flores-Miranda MC, Mazón-Suástegui JM (2015) Isolation and characterization of potential probiotic bacteria from pustulose ark (Anadara tuberculosa) suitable for shrimp farming. Lat Am J Aquat Res 43:123–136. https://doi.org/10.3856/vol43-issue1-fulltext-11
Sánchez-Ortiz AC, Angulo C, Luna-González A, Álvarez-Ruiz P, Mazón-Suástegui JM, Campa-Córdova AI (2016) Effect of mixed–Bacillus spp. isolated from pustulose ark Anadara tuberculosa on growth, survival, viral prevalence, and immune-related gene expression in shrimp Litopenaeus vannamei. Fish Shellfish Immunol 59:95–102
Guillard RRL (1973) Handbook of phycological methods. Cambridge ed Division rates London.
Benbrook CM (2002) Antibiotic Drug Use in US Aquaculture. Northwest Science and Environmental Policy Center Sandpoint, Idaho. https://www.iatp.org/documents/antibiotic-drug-use-in-us-aquaculture-1.
Campa-Córdova AI, González-Ocampo HA, Luna-González A, Mazón-Suástegui JM, Ascencio F (2009) Growth survival and superoxide dismutase activity in juvenile Crassostrea corteziensis (Hertlein, 1951) treated with probiotics. Hidrobiológica 19(2):151–157
Luis-Villaseñor IE, Campa-Córdova AI, Huerta-Aldaz N, Luna-González A, Mazón-Suástegui JM, Flores-Higuera F (2013) Effect of beneficial bacteria on larval culture of Pacific whiteleg shrimp. Litopenaeus vannamei Afr J Microbiol Res 7(27):3471–3478
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovet DL, Mirvaghefi AR, Shakouri M (2006) The effect of Bacillus spp bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252(2–4):516–524. https://doi.org/10.1016/j.aquaculture.2005.07.021
Hemaiswarya S, Raja R, Kumar R, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746. https://doi.org/10.1007/s11274-010-0632-z
Nicolas JL, Corre S, Gauthier G, Robert R, Ansquer D (1996) Bacterial problems associated with scallop (Pecten maximus) larval culture. Dis Aquat Org 27:67–76
Aguilar-Macías OL, Ojeda-Ramírez JJ, Campa-Córdova AI, Saucedo PE (2010) Evaluation of natural and commercial probiotics for improving growth and survival of the pearl oyster, Pinctada mazatlanica, during late hatchery and early field culturing. J World Aquac Soc 41:447–454. https://doi.org/10.1111/j.1749-7345.2010.00386.x
Campa-Córdova AI, Luna-González A, Ascencio F, Cortés-Jacinto E, Cáceres-Martínez CJ (2006) Effects of chloramphenicol, erythromycin, and furazolidone on growth of Isochrysis galbana and Chaetoceros gracilis. Aquaculture 260:145–150. https://doi.org/10.1016/J.Aquaculture.2006.06.014
Grossart HP, Czub G, Simon M (2006) Algae–bacteria interactions and their effects on aggregation and organic matter flux in the sea. Enviromental Microbiol 8:1074–1084. https://doi.org/10.1111/j.1462-2920.2006.00999.x
Grossart HP, Simon M (2007) Interactions of planktonic algae and bacteria: effects on algal growth and organic matter dynamics. Aquat Microb Ecol 47:163–176. https://doi.org/10.3354/ame047163
Toi HT, Boeckx P, Sorgeloos P, Bossier P, Van Stappen G (2014) Co-feeding of microalgae and bacteria may result in increased N assimilation in Artemia as compared to mono-diets, as demonstrated by a 15N isotope uptake laboratory study. Aquaculture 422–423:109–114. https://doi.org/10.1016/j.aquaculture.2013.12.005
De Paiva-Maia E, Alves-Modesto G, Otavio-Brito L, Olivera A, Vasconcelos-Gesteira TC (2013) Effect of a commercial probiotic on bacterial and phytoplankton concentration in intensive shrimp farming (Litopenaeus vannamei) recirculation systems. Lat Am J Aquat Res 41:126–137. https://doi.org/10.3856/vol41-issue1-fulltext-10
Pacheco-Vega JM, Cadena-Roa MA, Leyva-Flores JA, Zavala-Leal OI, Pérez-Bravo E, Ruiz-Velazco JMJ (2018) Effect of isolated bacteria and microalgae on the biofloc characteristics in the Pacific white shrimp culture. Aquac Report. https://doi.org/10.1016/j.aqrep.2018.05.003
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol Adv 29:896–907. https://doi.org/10.1016/j.biotechadv.2011.07.009
Molina-Cárdenas CA, Sánchez-Saavedra MP (2017) Inhibitory effect of benthic diatom species on three aquaculture pathogenic vibrios. Algal Res 27:131–139. https://doi.org/10.1016/j.algal.2017.09.004
Nemutanzhela ME, Roets Y, Gardiner N, Lalloo R (2014) The use and benefits of Bacillus based biological agents in aquaculture. In: Hernandez-Vergara M. (ed.) Sustainable aquaculture techniques. Murcia Spain.
Timmerman H, Koning C, Mulder L, Rombouts F, Beynen A (2004) Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int J Food Microbiol 96(3):219–233
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28(2):214–220
Luis-Villaseñor IE, Macías-Rodríguez ME, Gómez-Gil B, Ascencio-Valle F, Campa-Córdova ÁI (2011) Beneficial effects of four Bacillus strains on the larval cultivation of Litopenaeus vannamei. Aquaculture 321:136–144. https://doi.org/10.1016/j.aquaculture.2011.08.036
Setyati WA, Martani E, Zainuddin M (2014) Selection, identification and optimization of the growth water probiotic consortium of mangrove ecosystems as bioremediation and biocontrol in shrimp ponds. JPHPI 17(3):243–253. https://doi.org/10.17844/jphpi.v17i3.8913
Newaj-Fyzul A, Al-Harbi AH, Austin B (2014) Review : Developments in the use of probiotics for disease control in aquaculture. Aquaculture 431:1–11. https://doi.org/10.1016/j.aquaculture.2013.08.026
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25:128–136. https://doi.org/10.1016/j.fsi.2008.03.013
Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, Nejat N (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 33:683–689. https://doi.org/10.1016/j.fsi.2012.05.027
Dhama K, Tiwari R, Khan RU, Chakraborty S, Gopi M, Karthik K, Saminathan M, Desingu PA, Sunkara LT (2014) Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: the trends and advances-A review. Int J Pharmacol 10:129–159
Silva-Aciares F, Moraga D, Auffret M, Tanguy A, Riquelme C (2013) Transcriptomic and cellular response to bacterial challenge (pathogenic Vibrio parahaemolyticus) in farmed juvenile Haliotis rufescens fed with or without probiotic diet. J Invertebr Pathol 113:163–176. https://doi.org/10.1016/j.jip.2013.03.004
Acknowledgements
The authors thank CIBNOR staff Delfino Barajas-Frías and Pablo Ormart-Castro for hatchery-rearing of larvae and juveniles; Julian Garzón-Favela for provision of microalgae; Maria Del Carmen Rodriguez Jaramillo for technical support with the fluorescence microscope and Diana Fisher for editorial services. Acuacultura Robles S.P.R. of R.I. donated oyster specimens. Funding was provided by CIBNOR, SEP-CONACYT (243532) and PROINNOVA–CONACYT (199788) grants. First author was recipient of a doctoral fellowship from CONACYT (209357).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Ortiz, A.C., Mazón-Suástegui, J.M., del C. Flores-Miranda, M. et al. Probiotic Bacterium and Microalga Interaction on Rearing Kumamoto Oyster Crassostrea sikamea Spat. Curr Microbiol 77, 2758–2765 (2020). https://doi.org/10.1007/s00284-020-02076-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02076-2