Abstract
Phytopathogens causing mycotoxicoses in maize are a potential threat to grain quality and availability in many maize producing countries including South Africa. The use of natural biological agents for controlling maize fusariosis and many other such plant diseases, as opposed to the more traditional use of chemicals, is growing in popularity globally, as a greater emphasis gets placed on public health. In this study, nine Fusarium inhibiting isolates representing a subset of 200 native Pseudomonas isolates from the maize rhizosphere of 10 different farms in the North West Province of South Africa were further evaluated for their in vitro biocontrol potential. Although few of the isolates (PS1.1, PS1.22, PS2.2, PS6.4, PS6.8, PS7.2, PS8.3, PS8.6, and PS9.1) had impressive in vitro biosuppressive effects against Fusarium graminearum and Fusarium culmorum, while also producing biosurfactants, only isolate HARBPS9.1 showed consistent antifungal capacity along with maize seed bioprotection capability. The candidate antagonist HARBPS9.1 was molecularly characterized as a Pseudomonas fulva strain and was found to harbor multiple functional biosynthetic gene clusters after FTIR, NMR and ESI-Q-TOF-MS metabolomics investigation. P. fulva HARBPS9.1 bioprotective mechanism is attributed to the possible presence of hydrogen cyanide, pyrrolnitrin, and rhamnolipids.
Similar content being viewed by others

Introduction
Interdisciplinary research involving various environmental strains of the Gram-negative, oxidase positive, catalase negative and aerobic rod-shaped, Pseudomonas genera has been ongoing for over 30 years. This is due to their metabolic diversity and the vast number of organic compounds they are able to metabolize. Presently, the total number of species within the genus Pseudomonas is debatable and could be well over 200 species with over 70 novel species added in the past year (Peix et al. 2018). The genus was formally grouped into nine major categories that include: P. syringae, P. fluorescens, P. oleovorans, P. lutea, P. anguilliseptica, P. putida, P. straminea, P. stutzeri, and P. aeruginosa (Gomila et al. 2015; Mulet et al. 2010). However, based on the influx of genomic data the taxonomic database is on a steady progressive update. These organisms show immense promise for use in plant growth promotion, biocontrol, and in the biofertilizer industry (Frapolli et al. 2007; Yanes and Bajsa 2016; Raaijmakers et al. 2008).
The fluorescent Pseudomonas strains have been shown to contribute positively to the disease suppressive capacity of soils, and when used as microbial inoculants, have protected plants from infection by soil-borne phytopathogens (Michelsen and Stougaard 2010). They are known to give such protection by a number of mechanisms, including but not limited to: competition, antibiosis, and inducing a systemic resistance against plant pathogens by producing diverse antimicrobial secondary metabolites (Raaijmakers et al. 2006; Pliego et al. 2011). Equally, plant-associated Pseudomonas strains have been reported to produce lytic enzymes, antibiotics (phenazines, pyrrolnitrin, 2, 4-diacetylphloroglucinol Phl), pyoluteorin (Plt)), siderophores, cyclic lipopeptides (amphisin, viscosinamide, and tensin), volatiles, rhamnolipids and hydrogen cyanide (HCN) (Raaijmakers et al. 2008). The roles of these antimicrobial metabolites in the ability of several Pseudomonas spp. to protect crop have also been fully described (Hassan et al. 2011; Müller et al. 2016).
Presently, the application of diverse beneficial rhizobacteria as bioinoculants for alleviating crop diseases caused by mycotoxigenic pathogens including Fusarium members, is generating considerable attention (Cordero et al. 2012; Vacheron et al. 2016) and reports of excellent biocontrol activities attributed to the genus Pseudomonas in this regard are available (Babalola et al. 2007; Hernández-León et al. 2014). Members of the Fusarium graminearum (Fg) species complex (FGSC) have been implicated in cereal grain fusariosis outbreaks worldwide (Summerell and Leslie 2011); additionally, Fg contributes largely to maize fusariosis in South Africa thereby affecting the yield and availability of the staple crop in major households (Boutigny et al. 2012; Boutigny et al. 2011; Boutigny et al. 2013). Mycotoxin (zearalenone and deoxynivalenol) contamination of major cereal grains by Fg is a serious environmental threat and has led to huge economic losses during pre-harvest and post-harvest seasons (Lamprecht et al. 2011; Janse van Rensburg et al. 2015; Boutigny et al. 2011).
Efforts to alleviate these challenges posed to food availability by mycotoxigenic phytopathogens using alternative and or environmentally friendly plant disease management approaches such as biological control are currently being explored (Abiala et al. 2015; Bardin et al. 2015; Mngqawa et al. 2016). The growing awareness of the environmental threat of non-biodegradable chemical fungicides and the resistance exerted by etiological agents of fusariosis (e.g. Fg and F. verticillioides) to synthetic pesticides in planta (Sevastos et al. 2018; Sevastos et al. 2016; Hou et al. 2018) has further led crop scientists to seek for these supplementary or alternative means of crop protection. Our goal in this present work was to select for an indigenous rhizobacteria Pseudomonas strain possessing bioprotective potential that could later be exploited for in planta use.
The effectiveness of a microbial bioinoculant (used as plant growth promoting or biocontrol agent) is determined by its persistence and survivability in the environment (Babalola 2010). Biosurfactants (surface-active agents with both lipophilic and hydrophilic moieties) give plant growth promoting microbes competitive advantage (Rivardo et al. 2009) and because biosurfactants have eco-friendly properties, there has been growing evidence to support their agro-industrial and biotechnological application (Santos et al. 2016; Naughton et al. 2019; Mnif and Ghribi 2015). Thus, the main objectives of the study were to: (a) isolate native Pseudomonas strains from the maize rhizosphere and select those with activity against Fusarium, (b) determine their biosurfactant production potential, (c) select an isolate from among the candidate pseudomonads with bioprotective ability against Fusarium maize seed proliferation, and (d) chemically and molecularly characterize the isolate to identify its antifungal mechanisms.
Materials and methods
Sampling area and collection from the rhizosphere
The North West Province of South Africa covers 28,206 km2, with a temperature variation of between 17 °C and 31 °C during the summer months and between 3 °C and 21 °C during the winter month. Furthermore, this province typically has an average rainfall of approximately 360 mm. Depending on the width of the maize plot, 20–30 g of rhizospheric soil was sampled randomly from four maize rows 15–25 m apart, in each plot, from 10 maize farms in the North West province. Maize plants were collected and the roots lightly shaken in order to remove the loosely attached soil. The remaining soil adhering to the roots of the plants was considered to be the rhizosphere soil. The rhizospheric soil samples collected from each plot were subsequently pooled for each location before analysis (i.e. compound representative rhizospheric soil was analyzed per plot) and 10 different soil samples were obtained in total from the various different maize farms.
Isolation of Pseudomonas spp. from rhizospheric soil
Luria–Bertani (LB) broth (Sigma Aldrich (L3522) and Pseudomonas agar (Sigma Aldrich (P1852)) plates with cetrinix supplement (Sigma Aldrich (C8721)) were prepared according to manufacturer’s instructions. Five grams of each soil sample was inoculated into 45 mL of LB broth and incubated at 35 °C for 16 h with continuous shaking at 150 rpm. A calibrated inoculating loop was used to streak onto the surface of 20 Pseudomonas agar with cetrinix supplement. Ten isolates of each morphotype were then randomly selected from each plate based on the distinct characteristics exhibited on the selective-differential agar used for culture. Distinct colonies (of 200 isolates) were carefully selected and preserved at −80 °C in LB broth with 15% glycerol (v/v). Fifteen milliliters of each isolate in LB broth and agar slant was also kept at 4 °C as a working culture.
The fungal pathogens Fg and Fusarium culmorum (Fcul) provided by Dr. Claire Prigent Combaret (UMR CNRS 5557) Microbial Ecology of Lyon, University Lyon 1, France and Prof Cristina Cruz CE3C, Centre for Ecology, Evolution and Environmental Changes, Faculdade de Ciências da Universidade de Lisboa, Portugal, respectively, were maintained on Potato Dextrose Agar (PDA Sigma Aldrich P2182) plates. Bacillus velezensis NWUMFkBS10.5 (accession no.: NZ_NITU01000037.1; GCF_002204665.1), previously identified as a Fusarium antagonist (Adeniji et al. 2018), was also subcultured and maintained on LB plates for later use as a positive control during the maize seed bioprotection assay.
Screening of Pseudomonas isolates for anti-Fusarium activity
Preliminary anti-Fusarium activity
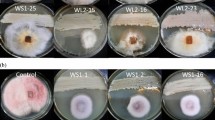
A preliminary multiple confrontation dual culture tests to detect the antagonistic activities of 200 Pseudomonas isolates against Fg, was carried out. A 5 mm diameter plug from an actively growing (7-day-old) mycelial culture of Fg was placed in the center of freshly prepared PDA plates (90 mm). From the 200 Pseudomonas isolates initially selected, six fresh colonies from 24 h LB (Sigma Aldrich L3147) agar culture were streaked circularly (equidistance 1.5 cm) on each PDA plate at a distance of 1.5 cm from the edge of the plate, using a sterile inoculating loop. Control plates consisted of Fg placed on PDA alone. The plates were incubated at 28 °C for 7 days. Thereafter, isolates exhibiting strong inhibition were selected for the antifungal confirmatory test.
Confirmatory anti-Fusarium activity
To confirm the inhibitory potentials of the bacterial antagonists, a single bacterial antagonist each (from the selected subset in the preliminary anti-Fusarium activity section) was pre-inoculated at the center of the PDA 3 days before both fungal agar plugs were inoculated on opposite sides. The petri-dishes for the inhibition assay were prepared as described above. The antagonistic effect was determined by measuring the zones of inhibition (mm). Experiments were repeated three times and the values recorded as the means of three replicates. Only nine isolates (a subset of the pseudomonads from the preliminary anti-Fusarium section), with impressive inhibition zones against the two pathogens in these screening plates, were selected for further analysis.
Screening for biosurfactant production potential
The capacity of the nine isolates (from the confirmatory anti-Fusarium activity section) to lyse erythrocytes was determined using a previously described method (Chakraborty et al. 2014), with slight modifications. Plates of blood agar (HiMedia, India) were streaked with a loopful of a fresh colony of each isolate and subsequently incubated for 48 to 72 h at 37 °C. The plates were observed for lyses around the colonies. The isolates were further subjected to a modified “drop collapse test” (Yanes et al. 2012). Briefly, 5 μL of cell-free supernatant from an overnight LB broth culture of each isolate, was placed in the center of a 96 well plate previously coated with 2 μL of commercial test substances consisting of motor engine oil, hexadecane, vegetable oil, paraffin oil, kerosene, and subsequently equilibrated for 2 h. After 1 min incubation, the test was scored visually and recorded negative if the drop remained beaded or positive if the drop collapsed. The test was repeated in triplicate. This was done in comparison to the negative control of water and LB broth.
HARBPS9.1 bioprotection of maize seed against F. graminearum and F. culmorum proliferation
Isolate HARBPS9.1 was selected for maize bioprotection assay, metabolite extraction, chemical and molecular characterization due to its unique antifungal activity and its biosurfactant production potential.
Surface disinfection of maize seeds
Maize seeds variety DKC 73–72 (200 g) were washed in sterile distilled water to remove any fungicide, soaked for 5 min in a sodium hypochlorite solution (0.75%), rinsed five times with sterile distilled water and soaked in the fifth wash. The fifth wash was inoculated on nutrient agar to ascertain the disinfection efficiency and surface disinfection was checked by the absence of colony forming unit on the Nutrient agar (NA).
HARBPS9.1 bioprotection of maize seeds
Using a modified experimental assay (Rahman et al. 2016), the bioprotective ability of HARBPS9.1 on sterile maize seeds against Fg and Fcul proliferation was determined. B. velezensis NWUMFkBS10.5 served as a positive control treatment. Briefly, a loopful of each bacterial isolate from a 24 h LB agar fresh culture was inoculated in 100 ml LB broth flask and incubated overnight at 37 °C with continuous shaking at 180 rpm. After overnight culture, sterile maize seeds were submerged in the LB bacteria broth (diluted to OD 0.5:600 nm), and incubated for 18 h. Flasks were drained aseptically and air dried under laminar flow for 1 h and kept for further analysis. From the air-dried bacterized seeds, five grains were also aseptically removed and placed in a row at the center of PDA agar plates, and then 5 mm agar plugs of both Fg and Fcul were inoculated at opposite sides of the grains/plate. Plates were incubated at 28 °C for 7 days in the dark and suppression of fungal mycelia was assessed visually. The numbers of sprouted seeds (with coleoptile) and seeds covered with mycelia were recorded. The length of the radicle/shoot of the sprouted grains was also recorded. Untreated seeds were used as a negative control. The experiment was done in four replicates and repeated three times.
Antifungal activity of the HARBPS9.1 cell-free supernatant and lyophilized extract
Extraction and collection of HARBPS9.1 culture filtrate
The production and purification of the active metabolites were performed as described by Gond et al. (2015), with slight modifications. HARBPS9.1 was grown in 1 L of LB-broth at 30 °C, with continuous shaking at 200 rpm for 72 h. The cells were harvested by centrifugation at 13,000×g for 15 min, and the culture supernatant was filter sterilized through 0.22 μm nitrocellulose membranes (Millipore Corporation, Bedford, MA, U.S.A.) filters, in order to obtain cell-free supernatants, of which 100 mL was stored for antifungal test. Isolate HARBPS9.1 was further grown in 1 L of LB-broth at 30 °C with constant shaking at 200 rpm for 4 days. After fermentation, the cell filtrate was collected by centrifugation, at 6000 rpm for 15 min at 4 °C and the supernatant was acid precipitated by adjusting to pH 2.0 with 6 M HCl. After overnight incubation at 4 °C, the precipitate was centrifuged at 8000 rpm at 4 °C, for 15 min and the pellet was dissolved in methanol-water (50:50) and filtered through a 0.22 μm PTFE membrane in order to remove any particulates. The filtrate was subsequently concentrated using a vacuum evaporator at 45 °C, lyophilized, and stored in −80 °C until needed.
HARBPS9.1 culture filtrate antifungal activity
The antifungal activity of HARBPS9.1 cell-free supernatant and lyophilized extract was determined by disc diffusion as described by Chen et al. (2010), with slight modifications. Briefly, sterile filter paper disc impregnated with 60 μL of the cell-free supernatant of HARBPS9.1 was placed at the far edge of a PDA plate, and a 5 mm agar plug of Fusarium pathogens (Fg and Fcul) was transferred to the opposite edge of the PDA plates. Nystatin (30 μg/disc) was used as a control, and after 7 days of incubation and observation of plates at 25 °C, zones of inhibition were recorded. The lyophilized extract from −80 °C was dissolved in phosphate buffered saline (PBS) (pH 7.5) to a concentration of 10 mg/mL and serially diluted to varying concentrations of 100, 90, 70, 50, 30 μg/mL. Sterile discs made from punctured filter paper were impregnated with 40 μL of each solution and allowed to dry. Impregnated discs were subsequently placed on the periphery of the freshly prepared PDA plate, containing a 5 mm disc of the fungal pathogens (Fg and Fcul). The plates consisting of three replicates were incubated for 5 days at 28 °C and antifungal activity was determined by the inhibition of microbial growth around the disc. Recordings were taken if zones of inhibition were spotted from the border of the disc to the perimeter of the visible pathogen.
Characterization of HARBPS9.1 culture filtrate by FTIR and micro-TOF-MS analysis
Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectroscopy identifies the type of chemical bonds and functional groups in compounds and it is useful in elucidating the components contained in a sample. The lyophilized extract, HARBPS9.1 (10 mg) was analyzed with a Transform Alpha (FT-IR) KBr integrated spectrometer (Bruker). Spectra were collected at 400 to 4000 wavenumbers (cm−1) with an average of 32 scans. The OPUS spectroscopy software was used to collate and view the absorption peaks.
Micro-TOF-MS analysis of the P. fulva HARBPS9.1 metabolite extract
A high-resolution mass spectrum was obtained for the purified sample extract HARBPS9.1, using an Applied Biosystems 4800 Plus Micro-TOF/TOF analyzer (AB Sciex, USA), operated in the positive ion mode, with an accelerating voltage of 20 kV, 337 nm nitrogen laser for ionization, and a-cyano-4-hydroxycinnamic acid for the matrix. Bruker compass data analysis was used to process the mass spectrometry data while molecular weights and formulae were characterized by mass spectrum smart formula tools.
Extraction of HARBPS9.1 genomic DNA
A Zymo Research ZR Soil Microbe DNA Miniprep genomic isolation kit (Epigenetics) was used for the extraction of the genomic DNA of HARBPS9.1, and Nanodrop 1000 (Thermo Scientific, Wilmington, DE, USA) was used to assess its DNA quantity and quality. The extracted DNA was used for subsequent polymerase chain reaction (PCR) analysis.
Molecular characterization and biosynthetic gene screening of HARBPS9.1
Identification of the isolate HARBPS9.1 was done by amplifying the 16S rDNA gene in its genomic DNA as previously described by Widmer et al. (1998). Also, isolate HARBPS9.1 was screened for genes involved in the biosynthesis of hydrogen cyanide (hcnBC), 2,4-diacetylphloroglucinol (phlD), acc deaminase (acc), phenazine-1-carboxylic acid (phzCD), indole pyruvate decarboxylase (ipdc), pyoluteorin (plt), and pyrrolnitrin (prnD and prnC) respectively following protocols previously established by Kim et al. (2013). PCR with of 25 μL reaction mixture containing 1.5–2.5 μg of template DNA; 1 μL of primer, 12.5 μL OneTaq quick-load 2X master mix with standard buffer (NewEnglandBiolabs NEB), and 9.5–10.5 μL nuclease-free water, was done using a Bio-Rad thermocycler. Whitehead Scientific - Integrated DNA Technologies, synthesized all the primers used in the PCR amplification. The PCR amplicons were analyzed by electrophoresis in 1% (w/v) agarose gel and band sizes were determined using 1 kb molecular marker (Thermo Fisher Scientific). The gel containing 10 μg/mL ethidium bromide (Bio-Rad) were photo-visualized using a gel documentation system (Gel Doc 2000, Bio-Rad) to confirm the expected size of the PCR products. The PCR products were purified using a PCR purification kit (NucleoSpin Microbial DNA, Macherey-Nagel) and sent to Inqaba Biotec (Pretoria, South Africa) for sequencing.
Phylogenetic analysis
The HARBPS9.1 16S rDNA sequence was submitted to the Genbank and BLAST (Basic Local Alignment Search Tool) searched on the NCBI GenBank website (using default settings) to identify highly similar sequences. The sequence was compared with 14 other 16S rDNA partial sequences as described by Uchino et al. (2001) and 10 other related out-groups retrieved from the NCBI/GenBank data library. The BioEdit Sequence Alignment Editor software (v7.0.5.3; (Hall and BioEdit 1999)) and Multiple alignment program for amino acid or nucleotide sequences (MAFFT) online software (v7; default settings; (Katoh et al. 2017)) were used for sequence alignment. A phylogenetic tree was computed using Molecular Evolutionary Genetic Analysis (MEGA X: v10.0.5; (Kumar et al. 2018)).
Statistical analysis
A Multivariate General linear model was used to analyze treatment means and inhibition rates. Least significant difference test (LSD), Duncan multiple test and Student-Newman-Keuls (SNK) test were used to compare observed means, pathogen-antagonist relationship, treatment effects, and effect of conditions of inoculation using SPSS statistical package (version 22) at the significance level of 5%.
Results
Cultural characterization and identification of the selected isolates
From the 200 maize rhizobacterial isolates selected from the chromogenic agar, five isolates (PS2.2, PS7.2, PS1.22 PS6.4, and PS6.8) showed yellow fluorescent coloration, while isolate PS9.1 showed a characteristic creamy colony. (Figure S1). However, none of the isolates showed the cultural characteristics of the reference strain (Pseudomonas aeruginosa) as indicated by the manufacturer. Additionally, isolates PS1.22 and PS9.1 were the only mucoid isolates on the Pseudomonas agar. Table 1 shows the geographical zones where sampling was done. The selectivity of the chromogenic agar allowed us to tentatively assign the isolates that tested negative to Gram reaction, positive to oxidase and catalase tests in the Pseudomonas genus.
Anti-Fusarium activity and inhibition of fungal mycelia by Pseudomonas isolates
Only 9 isolates (PS1.1, PS1.22, PS2.2, PS6.4, PS6.8, PS8.3, PS8.6, PS9.1, and PS7.2) were selected based on their antifungal inhibitory potential (Fig. 1). Overall, isolate PS9.1 showed consistent inhibition against the 2 pathogens (Table 2) and Fcul was more susceptible to the majority of the isolates.
Biosurfactant production potential of Pseudomonas isolates
In this study, five of the nine isolates selected (PS7.2, PS1.22, PS8.3, PS9.1, and PS1.1) showed biosurfactant properties (Table 3). Considering that isolate PS9.1 (HARBPS9.1) which has biosurfactant properties also performed better against both Fusarium pathogens it was the only isolate that was molecularly characterized by 16S rDNA, assayed for maize bioprotective potential, chemically characterized, and screened for the presence of functional antibiotic genes, .
HARBPS9.1 bioprotection of maize seed against F. graminearum and F. culmorum proliferation
In the agar plate bioassay (Fig. 2 and Table S1), maize seeds treated with HARBPS9.1 and NWUMFkBS10.5 suppressed fungal mycelia growth on seed surfaces, while the control seeds were covered with mycelia. The bacterized seeds also appeared visually healthier compared to the untreated control seeds that showed a sign of rot.
HARBPS9.1 culture filtrate inhibit Fusarium pathogens
HARBPS9.1 cell-free supernatant exhibited antifungal activity against the fungal pathogens indicating that the supernatant contains bioactive substances. The inhibitory capacity exhibited was similar to that of the fungicide nystatin (Table S2). For the lyophilized extract antifungal activity, the efficacy of the extracts decreased proportionately, relative to the volume of PBS added and Fg was the most inhibited (29%) at the highest volume of the extract (100 μl) (Fig. 3).
HARBPS9.1 secondary metabolite extract contain antimicrobial compounds
The characteristic bands highlighted in the FTIR spectrum are that of a typical glycolipid metabolite. In Fig. S2, the absorption values located at 3040.5070, 2929.4794 and 2852.0966, corresponds to that of an aliphatic C-H stretch (CH2 and CH3), 1728.3626 and stretching vibrations at 1644.2508, 1216.9628 and 1021.8233 are typical of ester carbonyl groups (C=O), 1452.4759, 1728.3626 and 3279.3846 are characteristic of carboxylic acids and O-H stretch of hydroxyl groups. While the vibrations seen between 809.8615–466.6850 are characteristics of aromatic groups. The molecular ions m/z ratios of 107.0421, 186.1040, 266.0029, 439.2932, 530.2988, 606.7616, 652.4002, 743.3992, 750.4088, 1058.6804, 1058.6740, 1124.5941 and 1463.8096, in the Micro-TOF-MS spectra are consistent with that of rhamnolipids and lipopeptides (Fig. S3a and S3b). The mass fragments at 107.0421, 186.1040 and 266.0029 m/z could be isotopic fragments of pyrrolnitrin (Fig. S3a), while the masses 1463.8096 m/z (Fig. S3a) and 1124.5941 m/z (Fig. S3b) may suggest the presence of two, as yet, unidentified compounds.
Molecular identification, biosynthetic gene screening and phylogenetic comparison of isolate HARBPS9.1
After the PCR amplification of the 16S rDNA (Psmn primer) and sequencing, the sequences were submitted to the Genbank and BLAST searched on the NCBI database (default settings). Identification of the HARBPS9.1 up to genus and species level was confirmed by the phylogenetic computational analysis. Our isolate HARBPS9.1 (accession: MF098600.1) shares a 100% identity with Pseudomonas fulva strain (accession: MF462916.1) and forms a clade with three other P. parafulva taxa (Fig. 4). Further results show that the biosynthetic genes responsible for the production of hydrogen cyanide and pyrrolnitrin were detected in DNA amplicon of P. fulva strain HARBPS9.1. DNA fragments of approximately 587 bp and 790 bp were amplified from our P. fulva HARBPS9.1 strain using the hydrogen cyanide-primer (Aca), targeting the hcnAB gene and pyrrolnitrin-primers, (PRND/PRNC) targeting the prnD/prnC gene respectively (Fig. 5). PCR products for 2, 4-diacetylphloroglucinol (phlD), acc deaminase (acc3), phenazine-1-carboxylic acid (phzCD), indole pyruvate decarboxylase (ipdc), and pyoluteorin (plt) were however not amplified.
Phylogenetic relatedness of Pseudomonas fulva HARBPS9.1 and several closely related species based on 16SrDNA gene sequences. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. Black and grey shaded circles with letter T represents the typed strain of P. parafulva and P. fulva respectively
Discussion
Although the in vitro dual culture prescreening of large numbers of microorganism for antibiosis is laborious, it is still largely considered as one of the most efficient and inexpensive methods for selecting candidate biocontrol agents from numerous promising strains (Pliego et al. 2011; Shehata et al. 2016; Adeniji and Babalola 2018). The nine selected maize rhizobacteria Pseudomonas isolates in this investigation showed appreciable in vitro biosuppression of toxigenic fungi (Fg and Fcul). Future study will however, evaluate their in planta biosuppressive abilities considering that Pseudomonas sp. remains one of the best-characterized plant growth promoting rhizobacteria (PGPR) and its many beneficial traits, has increased its commercial value.
The biosurfactant producing capability of the five anti-Fusarium candidates (PS7.2, PS1.22, PS8.3, PS9.1, and PS1.1) in this study was confirmed by the hemolysis and drop collapse assays. Soil-borne biosurfactant producing pseudomonads antagonizing multiple plant pathogens have been reported previously (Alsohim et al. 2014; Ben Belgacem et al. 2015). In contrast to chemically synthesized surfactants, biosurfactants (surface-active substances produced by diverse microbes) have become a more attractive option for biotechnological application due to their environmentally friendliness, biodegradability, and non-hazardousness (Soberón-Chávez and Maier 2011). Microbial surfactants are further endowed with diverse biological activities (e.g. antifungal, antiviral, anti-insecticidal, antibacterial, and hemolytic properties) (Mnif and Ghribi 2015). Therefore, apart from their bioprotective potential, the portion of the pseudomonads in this study with surface-active properties might also be useful in bioemulsification and other bioremediation studies.
Isolate HARBPS9.1 was selected for further analysis due to its unique antifungal activity against both Fusarium pathogens and its biosurfactant production potential. This could be a valuable property if the isolate is to be considered as a bioinoculant before seeding or during grain cultivation and as a preservative during grain storage. Comparatively, there was no observable significant difference in the bioprotective potential of the two rhizobacteria test strains (HARBPS9.1 and NWUMFkBS10.5) during the maize bioprotective assays. But when compared with the control seeds, both rhizobacteria strains suppressed fungal mycelia growth on the bacterized seed surfaces and enhanced the vigor of the growing seeds.
The FT-IR result of this study is consistent with previous reports confirming the presence of a glycolipid biosurfactant (Moussa et al. 2014; Borah et al. 2016) and for the ESI-Q-TOF MS result, signals corresponding to that produced by β-hydroxy fatty acids and rhamnose moieties previously reported in Pseudomonas strains (Price et al. 2009; Lotfabad et al. 2010; Moussa et al. 2014) were identified in the HARBPS9.1 extract. Several factors influence the accurate chemical characterization of microbial biomolecules. Previously published variations seen in the structural composition of some microbial-derived bioactive compounds, were reported to be largely influenced by the species and or strains of organisms investigated, fermentation conditions, extraction methods, and analytical equipments employed (Lotfabad et al. 2010; Hošková et al. 2013). In this investigation, the metabolomic probing of HARBPS9.1 cell-free secondary metabolite and lyophilized extract by FTIR and ESI-Q-TOF MS revealed the presence of surface-active compounds which was confirmed by the antifungal activity of the HARBPS9.1 cell-free supernatant and lyophilized extract. This is also a valuable attribute if HARBPS9.1 is to be applied in planta (e.g. as a pre-seeding bioinoculant or grain cultivation bioinoculant and as a stored grain preservative).
ESI-Q-TOF MS has successfully been used to characterize microbial biosurfactants such as rhamnolipids (Pereira et al. 2012). The latter consists of one or two conserved β-hydroxyl fatty acids chains, glycosidically linked to a rhamnosyl motif (mono-rhamnose or di-rhamnose unit) (Price et al. 2009; Pereira et al. 2012). Rhamnolipids are also characterized by the presence of one or two rhamnose molecules (rhamnose rings) bound to a hydrophobic fatty acid moiety (long hydrocarbon chains) (Abdel-Mawgoud et al. 2010; Moussa et al. 2014; S. Zhang et al. 2016). Previous reports have suggested that the differentiation of the various isomeric forms of rhamnolipids containing pseudomolecular ions are challenging (Perfumo et al. 2006; Abdel-Mawgoud et al. 2010). Comparison of the molecular ions detected in this study with previous literature, however, suggests that the masses 439.2932 m/z and 530.2988 m/z, represent moieties of the mono-rhamnolipids (Rha–C10–C8, Rha–C10–C10, Rha–C10–C12:1 and Rha–C10–C12), while the masses 606.7616 m/z, 652.4002 m/z, 743.3992, and 750.4088 represent the di-rhamnolipids (Rha2–C10–C8, Rha2–C10–C10, Rha2–C10–C12:1 and Rha2–C10–C12) moieties (Perfumo et al. 2006; Lotfabad et al. 2010; Pereira et al. 2012; S. Zhang et al. 2016). The mass fragments 1058.6804 m/z and 1058.6740 m/z shows similarity with that of antimicrobial lipopeptides (Nam et al. 2015; Janek et al. 2010; Jasim et al. 2016), while masses 1463.8096 m/z and 1124.5941 m/z suggest the presence of two, as yet, unidentified compounds. Price et al. (2007), however, report that masses similar to 1463.8096 m/z and 1124.5941 m/z, indicate the possible presence of a polymyxin and sclerosin respectively, both of which have proven antifungal properties.
Phylogenetics of HARBPS9.1 16S rDNA gene sequences identifies it as a P. fulva – clading with three other P. parafulva lineages. P. fulva and P. parafulva share high ecological and taxonomic similarities (Peña et al. 2016; Uchino et al. 2001). The few genome reports of the beneficial efficacy (biocontrol and plant-growth promoting properties) of this closed knitted species (Liu et al. 2015; Y. Zhang et al. 2018; Pokojska-Burdjiez et al. 2004; Kakembo and Lee 2019), further highlights the relevance of this study. Huang et al. (2018), earlier reported the superior role pyrrolnitrin plays in Pseudomonas chlororaphis strain G05 biosuppression of Fg, this study, documents a likely simultaneous production of hydrogen cyanide and pyrrolnitrin in the P. fulva specie. Additionally, the mass fragments at 107.0421, 186.1040 and 266.0029 m/z closely resembling pyrrolnitrin’s molecular ions (Chernin et al. 1996; Burkhead et al. 1994), is of interest, considering that biosynthetic genes responsible for the production of hydrogen cyanide and pyrrolnitrin were detected in DNA amplicon of P. fulva strain HARBPS9.1. The mechanisms of action and conditions for production of pyrrolnitrin (a lipopeptide which directly interferes with fungal plasma membrane) and hydrogen cyanide have been well elaborated (Santoyo et al. 2012; Santo et al. 1998; Chernin et al. 1996), therefore, the presence of the two aforementioned antimicrobials in the Pseudomonas isolate HARBPS9.1, makes it a valuable candidate to be considered for field biocontrol trials. This study further suggests that HARBPS9.1 may be utilizing different mechanisms against the fungal pathogens. Since maize fusariosis is systemic in nature and could be detected at pre-harvest and post-harvest seasons, the availability of a native beneficial microbe such as HARBPS9.1, as a bioinoculant for cereal cultivation and storage, could help alleviate the emergence or spread of the disease in South Africa.
Change history
06 August 2020
This erratum is to inform readers of an incorrect version of the article was published online first due to vendor errors.
References
Abdel-Mawgoud, A. M., Lépine, F., & Déziel, E. (2010). Rhamnolipids: Diversity of structures, microbial origins and roles. [journal article]. Applied Microbiology and Biotechnology, 86(5), 1323–1336. https://doi.org/10.1007/s00253-010-2498-2.
Abiala, M., Odebode, A., Hsu, S., & Blackwood, C. (2015). Phytobeneficial properties of bacteria isolated from the rhizosphere of maize in southwestern Nigerian soils. Applied and Environmental Microbiology, 81(14), 4736–4743.
Adeniji, A. A., Aremu, O. S., & Babalola, O. O. (2018). Selecting lipopeptide-producing, Fusarium-suppressing Bacillus spp.: Metabolomic and genomic probing of Bacillus velezensis NWUMFkBS10.5. MicrobiologyOpen, 0(0), e742. https://doi.org/10.1002/mbo3.742.
Adeniji, A. A., & Babalola, O. O. (2018). Tackling maize fusariosis: In search of Fusarium graminearum biosuppressors. [journal article]. Archives of Microbiology, 200(8), 1239–1255. https://doi.org/10.1007/s00203-018-1542-y.
Alsohim, A. S., Taylor, T. B., Barrett, G. A., Gallie, J., Zhang, X.-X., Altamirano-Junqueira, A. E., et al. (2014). The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environmental Microbiology, 16(7), 2267–2281. https://doi.org/10.1111/1462-2920.12469.
Babalola, O. O. (2010). Beneficial bacteria of agricultural importance. Biotechnology Letters, 32(11), 1559–1570.
Babalola, O. O., Berner, D., & Amusa, N. (2007). Evaluation of some bacterial isolates as germination stimulants of Striga hermonthica. African Journal of Agricultural Research, 2(1), 27–30.
Bardin, M., Ajouz, S., Comby, M., Lopez-Ferber, M., Graillot, B., Siegwart, M., et al. (2015). Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Frontiers in Plant Science, 6, 566.
Ben Belgacem, Z., Bijttebier, S., Verreth, C., Voorspoels, S., Van de Voorde, I., Aerts, G., et al. (2015). Biosurfactant production by Pseudomonas strains isolated from floral nectar. Journal of Applied Microbiology, 118(6), 1370–1384. https://doi.org/10.1111/jam.12799.
Borah, S. N., Goswami, D., Sarma, H. K., Cameotra, S. S., & Deka, S. (2016). Rhamnolipid biosurfactant against Fusarium verticillioides to control stalk and ear rot disease of maize. Frontiers in Microbiology, 7, 1505. https://doi.org/10.3389/fmicb.2016.01505.
Boutigny, A. L., Beukes, I., Small, I., Zühlke, S., Spiteller, M., Van Rensburg, B. J., et al. (2012). Quantitative detection of Fusarium pathogens and their mycotoxins in south African maize. Plant Pathology, 61(3), 522–531.
Boutigny, A. L., Ward, T. J., Ballois, N., Iancu, G., & Ioos, R. (2013). Diversity of the Fusarium graminearum species complex on French cereals. European Journal of Plant Pathology, 138(1), 133–148. https://doi.org/10.1007/s10658-013-0312-6.
Boutigny, A. L., Ward, T. J., Van Coller, G. J., Flett, B., Lamprecht, S. C., O’Donnell, K., et al. (2011). Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genetics and Biology, 48(9), 914–920. https://doi.org/10.1016/j.fgb.2011.05.005.
Burkhead, K. D., Schisler, D. A., & Slininger, P. J. (1994). Pyrrolnitrin production by biological control agent Pseudomonas cepacia B37W in culture and in colonized wounds of potatoes. Applied and Environmental Microbiology, 60(6), 2031–2039.
Chakraborty, J., Chakrabarti, S., & Das, S. (2014). Characterization and antimicrobial properties of lipopeptide biosurfactants produced by Bacillus subtilis SJ301 and Bacillus vallismortis JB201. Applied Biochemistry and Microbiology, 50(6), 609–618.
Chen, L., Wang, N., Wang, X., Hu, J., & Wang, S. (2010). Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresource Technology, 101(22), 8822–8827. https://doi.org/10.1016/j.biortech.2010.06.054.
Chernin, L., Brandis, A., Ismailov, Z., & Chet, I. (1996). Pyrrolnitrin production by an Enterobacter agglomerans strain with a broad spectrum of antagonistic activity towards fungal and bacterial phytopathogens. [journal article]. Current Microbiology, 32(4), 208–212. https://doi.org/10.1007/s002849900037.
Cordero, P., Cavigliasso, A., Príncipe, A., Godino, A., Jofré, E., Mori, G., et al. (2012). Genetic diversity and antifungal activity of native Pseudomonas isolated from maize plants grown in a central region of Argentina. Systematic and Applied Microbiology, 35(5), 342–351. https://doi.org/10.1016/j.syapm.2012.04.005.
Frapolli, M., Défago, G., & Moënne-Loccoz, Y. (2007). Multilocus sequence analysis of biocontrol fluorescent Pseudomonas spp. producing the antifungal compound 2,4-diacetylphloroglucinol. Environmental Microbiology, 9(8), 1939–1955. https://doi.org/10.1111/j.1462-2920.2007.01310.x.
Gomila, M., Peña, A., Mulet, M., Lalucat, J., & García-Valdés, E. (2015). Phylogenomics and systematics in Pseudomonas. Frontiers in Microbiology, 6(214). https://doi.org/10.3389/fmicb.2015.00214.
Gond, S. K., Bergen, M. S., Torres, M. S., & White Jr., J. F. (2015). Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiological Research, 172, 79–87. https://doi.org/10.1016/j.micres.2014.11.004.
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symposium series, 1999 (Vol. 41, pp. 95–98, Vol. 41): [London]: Information Retrieval Ltd., c1979-c2000.
Hassan, M. N., Afghan, S., & Hafeez, F. Y. (2011). Biological control of red rot in sugarcane by native pyoluteorin-producing Pseudomonas putida strain NH-50 under field conditions and its potential modes of action. Pest Management Science, 67(9), 1147–1154. https://doi.org/10.1002/ps.2165.
Hernández-León, R., Rojas-Solís, D., Contreras-Pérez, M., Ma del Orozco-Mosqueda, C., Macías-Rodríguez, L. I., & Reyes-de La Cruz, H. (2014). Antifungal and plant-growth promoting effects of diffusible and volatile sulfur-containing compounds produced by Pseudomonas fluorescens strains. Biological Control, 81, doi:https://doi.org/10.1016/j.biocontrol.2014.11.011,
Hošková, M., Schreiberová, O., Ježdík, R., Chudoba, J., Masák, J., Sigler, K., & Řezanka, T. (2013). Characterization of rhamnolipids produced by non-pathogenic Acinetobacter and Enterobacter bacteria. Bioresource Technology, 130, 510–516. https://doi.org/10.1016/j.biortech.2012.12.085.
Hou, Y.-P., Qu, X.-P., Mao, X.-W., Kuang, J., Duan, Y.-B., Song, X.-s., et al. (2018). Resistance mechanism of Fusarium fujikuroi to phenamacril in the field. Pest Management Science, 74(3), 607–616, doi:https://doi.org/10.1002/ps.4742.
Huang, R., Feng, Z., Chi, X., Sun, X., Lu, Y., Zhang, B., et al. (2018). Pyrrolnitrin is more essential than phenazines for Pseudomonas chlororaphis G05 in its suppression of Fusarium graminearum. Microbiological Research, 215, 55–64. https://doi.org/10.1016/j.micres.2018.06.008.
Janek, T., Łukaszewicz, M., Rezanka, T., & Krasowska, A. (2010). Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic archipelago of Svalbard. Bioresource Technology, 101(15), 6118–6123. https://doi.org/10.1016/j.biortech.2010.02.109.
Janse van Rensburg, B., McLaren, N. W., Flett, B. C., & Schoeman, A. (2015). Fumonisin producing Fusarium spp. and fumonisin contamination in commercial south African maize. [journal article]. European Journal of Plant Pathology, 141(3), 491–504. https://doi.org/10.1007/s10658-014-0558-7.
Jasim, B., Sreelakshmi, K., Mathew, J., & Radhakrishnan, E. (2016). Surfactin, Iturin, and Fengycin biosynthesis by endophytic Bacillus sp. from Bacopa monnieri. Microbial Ecology, 1–14.
Kakembo, D., & Lee, Y. H. (2019). Analysis of traits for biocontrol performance of Pseudomonas parafulva JBCS1880 against bacterial pustule in soybean plants. Biological Control, 134, 72–81. https://doi.org/10.1016/j.biocontrol.2019.04.006.
Katoh, K., Rozewicki, J., & Yamada, K. D. (2017). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108.
Kim, J. S., Mele, P. M., & Crowley, D. E. (2013). Application of PCR primer sets for detection of Pseudomonas sp. functional genes in the plant rhizosphere. Journal of Agricultural Chemistry and Environment, 2(1), 8–15.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549.
Lamprecht, S. C., Tewoldemedhin, Y. T., Botha, W. J., & Calitz, F. J. (2011). Fusarium graminearum species complex associated with maize crowns and roots in the Kwazulu-Natal province of South Africa. Plant Disease, 95(9), 1153–1158. https://doi.org/10.1094/PDIS-02-11-0083.
Liu, Q., Zhang, Y., Yu, N., Bi, Z., Zhu, A., Zhan, X., et al. (2015). Genome sequence of Pseudomonas parafulva CRS01-1, an antagonistic bacterium isolated from rice field. Journal of Biotechnology, 206, 89–90. https://doi.org/10.1016/j.jbiotec.2015.03.017.
Lotfabad, T. B., Abassi, H., Ahmadkhaniha, R., Roostaazad, R., Masoomi, F., Zahiri, H. S., et al. (2010). Structural characterization of a rhamnolipid-type biosurfactant produced by Pseudomonas aeruginosa MR01: Enhancement of di-rhamnolipid proportion using gamma irradiation. Colloids and Surfaces B: Biointerfaces, 81(2), 397–405. https://doi.org/10.1016/j.colsurfb.2010.06.026.
Michelsen, C. F., & Stougaard, P. (2010). A novel antifungal Pseudomonas fluorescens isolated from potato soils in Greenland. [journal article]. Current Microbiology, 62(4), 1185–1192. https://doi.org/10.1007/s00284-010-9846-4.
Mngqawa, P., Shephard, G. S., Green, I. R., Ngobeni, S. H., de Rijk, T. C., & Katerere, D. R. (2016). Mycotoxin contamination of home-grown maize in rural northern South Africa (Limpopo and Mpumalanga provinces). Food Additives & Contaminants: Part B, 9(1), 38–45. https://doi.org/10.1080/19393210.2015.1121928.
Mnif, I., & Ghribi, D. (2015). Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Peptide Science, 104(3), 129–147.
Moussa, T., Mohamed, M., & Samak, N. (2014). Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Brazilian Journal of Chemical Engineering, 31(4), 867–880.
Mulet, M., Lalucat, J., & García-Valdés, E. (2010). DNA sequence-based analysis of the Pseudomonas species. Environmental Microbiology, 12(6), 1513–1530. https://doi.org/10.1111/j.1462-2920.2010.02181.x.
Müller, T., Behrendt, U., Ruppel, S., von der Waydbrink, G., & Müller, M. E. H. (2016). Fluorescent pseudomonads in the phyllosphere of wheat: Potential antagonists against fungal phytopathogens. Current Microbiology, 72(4), 383–389. https://doi.org/10.1007/s00284-015-0966-8.
Nam, J., Jung, M. Y., Kim, P. I., Lee, H. B., Kim, S. W., & Lee, C. W. (2015). Structural characterization and temperature-dependent production of C17-fengycin B derived from Bacillus amyloliquefaciens subsp. plantarum BC32-1. [journal article]. Biotechnology and Bioprocess Engineering, 20(4), 708–713. https://doi.org/10.1007/s12257-015-0350-3.
Naughton, P. J., Marchant, R., Naughton, V., & Banat, I. M. (2019). Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. Journal of Applied Microbiology, 127(1), 12–28. https://doi.org/10.1111/jam.14243.
Peix, A., Ramírez-Bahena, M.-H., & Velázquez, E. (2018). The current status on the taxonomy of Pseudomonas revisited: An update. Infection, Genetics and Evolution, 57, 106–116. https://doi.org/10.1016/j.meegid.2017.10.026.
Peña, A., Busquets, A., Gomila, M., Mulet, M., Gomila, R. M., Reddy, T., et al. (2016). High quality draft genome sequences of Pseudomonas fulva DSM 17717 T, Pseudomonas parafulva DSM 17004 T and Pseudomonas cremoricolorata DSM 17059 T type strains. Standards in Genomic Sciences, 11(1), 55.
Pereira, J. F., Gudiña, E. J., Dória, M. L., Domingues, M. R., Rodrigues, L. R., Teoxeira, J. A., et al. (2012). Characterization by electrospray ionization and tandem mass spectrometry of rhamnolipids produced by two Pseudomonas aeruginosa strains isolated from Brazilian crude oil. European Journal of Mass Spectrometry, 18(4), 399–406.
Perfumo, A., Banat, I. M., Canganella, F., & Marchant, R. (2006). Rhamnolipid production by a novel thermophilic hydrocarbon-degrading Pseudomonas aeruginosa AP02-1. Applied Microbiology and Biotechnology, 72(1), 132–138.
Pliego, C., Ramos, C., Vicente, A., & Cazorla, F. (2011). Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant and Soil, 340, 505–520. https://doi.org/10.1007/s11104-010-0615-8.
Pokojska-Burdjiez, A., Strzelczyk, E., Dahm, H., & Li, C. (2004). Effect of endophytic bacterium Pseudomonas fulva on growth of pine seedlings (Pinus sylvestris), formation of mycorrhizae and protection against pathogens. Phytopathologia Polonica, 32, 33–47.
Price, N. P., Ray, K. J., Vermillion, K., & Kuo, T.-M. (2009). MALDI-TOF mass spectrometry of naturally occurring mixtures of monorhamnolipids and dirhamnolipids. Carbohydrate Research, 344(2), 204–209.
Price, N. P., Rooney, A. P., Swezey, J. L., Perry, E., & Cohan, F. M. (2007). Mass spectrometric analysis of lipopeptides from Bacillus strains isolated from diverse geographical locations. FEMS Microbiology Letters, 271(1), 83–89.
Raaijmakers, J. M., Bruijn, I., & Kock, M. J. (2006). Cyclic lipopeptide production by plant-associated Pseudomonas spp.: Diversity, activity, biosynthesis, and regulation. Molecular Plant Microbe Interaction, 19. https://doi.org/10.1094/mpmi-19-0699.
Raaijmakers, J. M., Paulitz, T. C., Steinberg, C., Alabouvette, C., & Moënne-Loccoz, Y. (2008). The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant and Soil, 321(1–2), 341–361. https://doi.org/10.1007/s11104-008-9568-6.
Rahman, M. E. M., Hossain, D. M., Suzuki, K., Shiiya, A., Suzuki, K., Dey, T. K., et al. (2016). Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. [journal article]. Australasian Plant Pathology, 45(1), 103–117. https://doi.org/10.1007/s13313-016-0397-4.
Rivardo, F., Turner, R. J., Allegrone, G., Ceri, H., & Martinotti, M. G. (2009). Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. [journal article]. Applied Microbiology and Biotechnology, 83(3), 541–553. https://doi.org/10.1007/s00253-009-1987-7.
Santo, R. D., Costi, R., Artico, M., Massa, S., Lampis, G., Deidda, D., et al. (1998). Pyrrolnitrin and related pyrroles endowed with antibacterial activities against Mycobacterium tuberculosis. Bioorganic and Medicinal Chemistry Letters, 8(20), 2931–2936. https://doi.org/10.1016/S0960-894X(98)00526-5.
Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2016). Biosurfactants: Multifunctional biomolecules of the 21st century. International Journal of Molecular Sciences, 17(3), 401.
Santoyo, G., Orozco-Mosqueda, M. D. C., & Govindappa, M. (2012). Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Science and Technology, 22(8), 855–872. https://doi.org/10.1080/09583157.2012.694413.
Sevastos, A., Kalampokis, I. F., Panagiotopoulou, A., Pelecanou, M., & Aliferis, K. A. (2018). Implication of Fusarium graminearum primary metabolism in its resistance to benzimidazole fungicides as revealed by 1H NMR metabolomics. Pesticide Biochemistry and Physiology, 148, 50–61. https://doi.org/10.1016/j.pestbp.2018.03.015.
Sevastos, A., Markoglou, A., Labrou, N. E., Flouri, F., & Malandrakis, A. (2016). Molecular characterization, fitness and mycotoxin production of Fusarium graminearum laboratory strains resistant to benzimidazoles. Pesticide Biochemistry and Physiology, 128, 1–9. https://doi.org/10.1016/j.pestbp.2015.10.004.
Shehata, H. R., Ettinger, C. L., Eisen, J. A., & Raizada, M. N. (2016). Genes required for the anti-fungal activity of a bacterial endophyte isolated from a corn landrace grown continuously by subsistence farmers since 1000 BC. Frontiers in Microbiology, 7, 1548. https://doi.org/10.3389/fmicb.2016.01548.
Soberón-Chávez, G., & Maier, R. M. (2011). Biosurfactants: A general overview. In Biosurfactants (pp. 1-11): Springer.
Summerell, B. A., & Leslie, J. F. (2011). Fifty years of Fusarium: How could nine species have ever been enough? [journal article]. Fungal Diversity, 50(1), 135–144. https://doi.org/10.1007/s13225-011-0132-y.
Uchino, M., Shida, O., Uchimura, T., & Komagata, K. (2001). Recharacterization of Pseudomonas fulva Iizuka and Komagata 1963, and proposals of Pseudomonas parafulva sp. nov. and Pseudomonas cremoricolorata sp. nov. The Journal of General and Applied Microbiology, 47(5), 247–261. https://doi.org/10.2323/jgam.47.247.
Vacheron, J., Moënne-Loccoz, Y., Dubost, A., Gonçalves-Martins, M., Muller, D., & Prigent-Combaret, C. (2016). Fluorescent Pseudomonas strains with only few plant-beneficial properties are favored in the maize rhizosphere. Frontiers in Plant Science, 7, 1212. https://doi.org/10.3389/fpls.2016.01212.
Widmer, F., Seidler, R. J., Gillevet, P. M., Watrud, L. S., & Di Giovanni, G. D. (1998). A highly selective PCR protocol for detecting 16S rRNA genes of the genus Pseudomonas (sensu stricto) in environmental samples. Applied and Environmental Microbiology, 64(7), 2545–2553.
Yanes, M. L., & Bajsa, N. (2016). Fluorescent Pseudomonas: A natural resource from soil to enhance crop growth and health. In S. Castro-Sowinski (Ed.), Microbial models: From environmental to industrial sustainability (pp. 323–349). Singapore: Springer Singapore.
Yanes, M. L., De La Fuente, L., Altier, N., & Arias, A. (2012). Characterization of native fluorescent Pseudomonas isolates associated with alfalfa roots in Uruguayan agroecosystems. Biological Control, 63(3), 287–295.
Zhang, S., Wu, W., Li, D., & Zheng, Q. (2016). Separation and purification of six biosurfactant rhamnolipids by high-speed countercurrent chromatography utilizing novel solvent selection method. Separation Science and Technology, 51(4), 673–680. https://doi.org/10.1080/01496395.2015.1117101.
Zhang, Y., Chen, P., Ye, G., Lin, H., Ren, D., Guo, L., et al. (2018). Complete Genome Sequence of Pseudomonas parafulva PRS09–11288, a Biocontrol Strain Produces the Antibiotic Phenazine-1-carboxylic Acid. Current Microbiology, 1–5.
Acknowledgments
The first author appreciates North-West University for post-doctoral fellowship. This work is based on the research supported by the National Research Foundation, South Africa (Grant UID: 81192).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All authors reviewed the manuscript and approved its submission.
Electronic supplementary material
ESM 1
(DOCX 996 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adeniji, A.A., Aremu, O.S., Loots, D.T. et al. Pseudomonas fulva HARBPS9.1: candidate anti-Fusarium agent in South Africa. Eur J Plant Pathol 157, 767–781 (2020). https://doi.org/10.1007/s10658-020-02035-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02035-4