Abstract
In the evolving field of adaptive MR guided radiotherapy, the need for dedicated procedures for acceptance and quality assurance is increasing. Research has been devoted to MR compatible dosimeters and phantoms, but to date no end-to-end test has been presented that covers an MRgRT workflow. Such an end-to-end test should comprise each step of the workflow and include all associated uncertainties. The purpose of this study was to investigate the usability of an anthropomorphic deformable and multimodal pelvis (ADAM-pelvis) phantom in combination with film dosimetry for end-to-end testing of an MRgRT adaptive workflow. The ADAM-pelvis phantom included surrogates for muscle tissue, adipose and bone, as well as deformable silicone organs mimicking a prostate patient. At the interfaces of the critical structures (bladder and rectum), small pieces of GafChromic EBT3 films were placed to measure delivered dose.
Pre-treatment MR imaging of the phantom was used to delineate the prostate, rectum and bladder and to generate a treatment plan to deliver 2 Gy to the prostate. Electron density (ED) map from CT imaging was used for dose calculation after deformable image registration (DIR) to the pre-treatment MR scan. At each fraction, bladder- and rectum filling was varied and a new adapted plan was generated. Dose calculation was performed using both a DIR-based ED map and a CT-based ED map after acquisition of a new CT scan of the phantom at each fraction. All dose calculations were performed taking into account the magnetic field.
A good agreement between measured and calculated dose was found using both, the CT-derived and the DIR-based ED map (2.0% and 2.8% dose difference, respectively). The gamma index pass-rate (3%/2 mm) varied from 96.4% to 100%.The ADAM-pelvis phantom was suitable for end-to-end testing in MR-guided radiotherapy and a very good agreement with the calculated dose was achieved.
Export citation and abstract BibTeX RIS
1. Introduction
Since its clinical introduction in 2014, the field of MR-guided radiation therapy (MRgRT) has rapidly evolved. Currently there are two systems introduced in the clinic, each one with its own characteristics (Mutic and Dempsey 2014, Tijssen et al 2017). Substantial clinical experience has been gained in this field during the past few years (Bohoudi et al 2017, 2019, Henke et al 2017, 2018, Palacios et al 2018, Werensteijn-Honingh et al 2019), but to date there has not been any report on a comprehensive end-to-end test for MRgRT. Studies on adaptive MRgRT have reported the use of step-and-shoot IMRT, of a deformed electron density map from a CT at each fraction for dose calculation, and also stressed the importance of the coincidence of the MR-RT isocenter (Bohoudi et al 2017, Green et al 2018, Woodings et al 2018). Implementation of these new techniques in radiotherapy requires examination of historically accepted dosimeters and at the same time devising new procedures and phantoms for commissioning and quality assurance (QA) (Barten et al 2018).
End-to-end tests have been extensively applied in the past in the field of radiotherapy in order to be confident on the accuracy and safety of the treatment procedure involved (Klein et al 2009). These tests focused on image guidance (Wang et al 2012, Wen et al 2018) and IMRT/VMAT dose delivery (Gillis et al 2005, Palta et al 2008, O'Daniel et al 2012). For this purpose anthropomorphic phantoms and also patient-specific phantoms were developed (Ehler et al 2014, Kamomae et al 2017, Cunningham et al 2018). In an MR-guided adaptive workflow, an ideal end-to-end test procedure should cover pre-treatment MR and CT imaging, delineation, treatment planning, inter- and intra-fraction motion (for mobile targets), set-up imaging, image registration, online dose calculation and treatment delivery.
This study investigates the use of an anthropomorphic deformable pelvis phantom (Niebuhr et al 2019) in combination with film dosimetry as an end-to-end test for MRgRT on an MR-guided unit at 0.35T. GafChromic EBT3 film dosimetry has been shown to be a valuable and reliable dosimeter at low magnetic fields (Delfs et al 2018, Barten et al 2018). The construction of the anthropomorphic pelvis phantom allowed to assess the treatment delivery accuracy in critical areas located at the simulated organs at risk (OAR) and accurately examine any dosimetric induced errors by deformable image registration.
2. Materials and methods
2.1. Anthropomorphic deformable and multimodal phantom
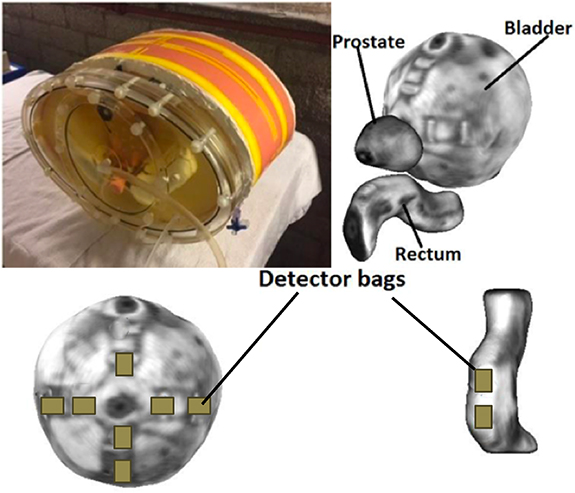
The ADAM-pelvis phantom, an anthropomorphic deformable multimodality pelvis phantom, was used for this study (Niebuhr et al 2019). The phantom is equipped with surrogates for inner and outer bone, muscle and adipose tissue, as well as deformable silicone organs mimicking the prostate, bladder and rectum. Materials and fillings of the phantom are optimized for contrast and electron density on both CT and MRI (Niebuhr et al 2016). By adding water or air, the filling of the bladder and rectum is adjustable. Furthermore, bladder and rectum are equipped with pockets at their surfaces which can hold small pieces of GafChromic films for dose measurements. Seven reproducible film positions were located at the bladder surface facing towards the prostate, two were located at the rectum surface (figure 1).
Figure 1. The ADAM-pelvis phantom and a MRI surface plot of the prostate, bladder and rectum (top). Film positions on the bladder and rectum (bottom).
Download figure:
Standard image High-resolution image2.2. End-to-end test workflow
The end-to-end test simulated the clinical workflow for MRgRT with online plan adaptation for prostate cancer (Tetar et al 2019). Using the ADAM-pelvis phantom, a total of four treatment fractions were simulated where a treatment plan was reoptimized at each fraction based on online MR image guidance setup in order to compensate for anatomical changes caused by bladder and rectum filling. Values for bladder and rectum filling in the phantom were recreated from derived clinical patient data to represent realistic scenarios. In these simulations, we used film dosimetry to measure the dose on the bladder- and rectum surfaces at the aforementioned film locations.
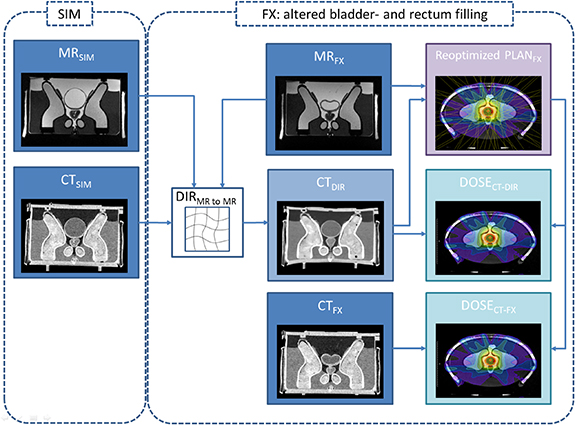
The phantom's bladder and rectum at baseline were filled with 320 cc water and 3 cc air, respectively. Figure 2 shows a schematic overview of the imaging, treatment planning and dose calculation workflow. For pretreatment a 1.0 mm × 1.0 mm × 1.25 mm resolution CT scan (GE LightSpeed RT) and a 1.5 mm × 1.5 mm × 1.5 mm resolution MR scan (MRIdian, true FISP, TR/TE of 3.83/1.62 ms, 60° flip angle) of the pelvis phantom were acquired prior to treatment planning (CTSIM and MRSIM). The CTSIM was rigidly registered to the MRSIM during treatment planning for dose calculation. Contouring of the prostate, bladder, rectum and bowel was performed on the MRSIM. Next, a baseline plan consisting of 15 equidistant step-and-shoot IMRT fields was generated to deliver 2 Gy per fraction to the PTV while sparing organs at risk according to our standard clinical protocol (Tetar et al 2019). The average number of segments per plan was 63 (range 48–80). Dose calculation was performed based on the electron density information of the CTSIM, using the ViewRay proprietary Monte Carlo algorithm with a statistical uncertainty of 1% on a 3 mm isotropic voxel size.
Figure 2. Imaging, treatment planning and dose calculation workflow. Pre-treatment consisted of an MRSIM and CTSIM procedure. At each fraction, the bladder/rectum volumes and densities were varied. The MRSIM with the contours for target and critical structures was deformable registered to the anatomy-of-the-day according to MRFX. The CTSIM underwent the same deformation generating a new CTDIR for that fraction. In addition, a new CT (CTFX) was acquired to compare the dose distributions generated with CTDIR and CTFX.
Download figure:
Standard image High-resolution imageA total of 4 treatment fractions were simulated and measured. Prior to each measurement, GafChromic EBT3 films were cut into 1 × 2 cm2 pieces and placed in the 9 available film pockets of the phantom. For the four fractions, the bladder filling was changed to 120, 98, 232 and 150 cc of water. The rectum filling was changed to 2 cc air, 2 cc water, 3 cc water and 2 cc air. After changing rectum and bladder filling and prior to treatment delivery, an additional CT-scan was acquired (CTFX). Without further changes, the phantom was then moved to the treatment machine where it was roughly positioned based on external lasers. Then, a 3D volumetric MR scan with the same protocol as for pretreatment was acquired (MRFX). After manual rigid alignment of MRFX to the CTV, MRSIM was subsequently registered to MRFX with deformable image registration (DIR) using the MRIdian integrated DIR algorithm. The deformation matrix was applied to CTSIM, resulting in a warped CT for that particular fraction (CTDIR). Based on the actual anatomy and using the electron density information from CTDIR, a new plan (PLANFX) was made by reoptimizing the base plan maintaining its original beam angles and optimization objectives. The 3D dose distribution (DOSECT-DIR) was exported for later analysis. After delivery of PLANFX, all films were extracted from the phantom and CTFX was imported into the treatment planning software (TPS), rigidly registered with MRFX and used to calculate a second dose distribution for PLANFX (DOSECT-FX) for comparison with DOSECT-DIR.
2.3. Film dosimetry
GafChromic EBT3 films (Lot #08251503) were cut into 1 × 2 cm2 pieces to fit in the pockets of the ADAM-pelvis phantom. Care was taken to mark out the (portrait) orientation of the film pieces. All films underwent the same dosimetric analysis irrespective of their orientation with the magnetic field, which was determined by their position in the phantom.
Two film calibration curves were created on the MRIdian in water under reference conditions (depth 5 cm, SSD 100 cm, field size 10.5 × 10.5 cm2). Films were digitized using a transmission flatbed scanner (Epson Expression 1680 Pro). All films were scanned in portrait mode along the central axis of the scanner in order to avoid lateral scanner artifacts (Battum and Hoffmans 2009). Film transmission values were translated to absolute dose (DOSEFILM) by means of a triple-color calibration method and generating a calibration curve with Matlab (R2015b, Mathworks) (Mayer et al 2012). In order to minimize post-exposure growth effects, a waiting period of approximately 24 h between irradiation and scanning was followed for all films (van Battum et al 2008).
The second set of calibration films was exposed to 5 subsequent CT scans using the same scanning protocols as used for CTFX. Following the procedure described above, these films were calibrated to dose in order asses the dose contribution from the CT scans. An average increase in dose of 4.4 cGy per scan (22.2 cGy for all 5 CT scans) was observed and this value was subtracted from all individual measurements.
2.4. Data analysis and evaluation
The 3D dose distributions (DOSECT-DIR and DOSECT-FX) and MRFX for each fraction were exported to 3D Slicer (version 4.8, www.3dslicer.org). Using the MRFX image data the film pockets were localized and the corresponding 2D dose planes extracted.
Dose measurements and calculations were imported in OmniPro I'mRT (version 1.7b, IBA Dosimetry) for data analysis. Films were registered to dose calculations based on the film edges. Dose readouts from films and dose calculations were cropped in order to avoid artifacts on the film edges. Relative and absolute average dose difference per cropped film was assessed. A two-tailed single and paired Wilcoxon signed rank test was used to evaluate the relative and absolute dose differences between the film dose readouts and the exported 2D dose planes (Rstudio, version 1.1.456). The Pearson correlation coefficient was evaluated for all data sets. Furthermore, gamma evaluation was performed and the gamma index calculated for DOSECT-DIR and DOSEFILM using 3% (global)/2 mm thresholds (Low 2002). Prior to gamma calculation, no normalization or scaling was applied to the measured dose distributions. The gamma pass-rate as well as the average gamma per delivery fraction for all film measurements was calculated.
3. Results
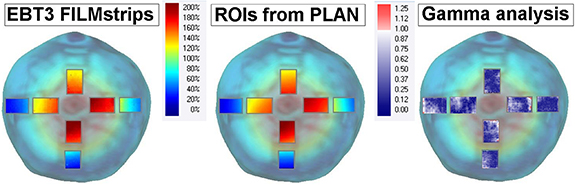
Figure 3 shows film measurements, 2D dose distributions extracted from treatment planning and their corresponding gamma evaluation for the regions of interest located at the bladder surface. The average measured dose in all films ranged from 30 to 208 cGy (15%–104% of prescribed dose). Depending on its location, the film was hence exposed to a high or low dose area and in some instances, also to a steep dose gradient.
Figure 3. Dose measurements, calculations (DOSECT-DIR) and their gamma evaluations for one fraction projected on the bladder surface.
Download figure:
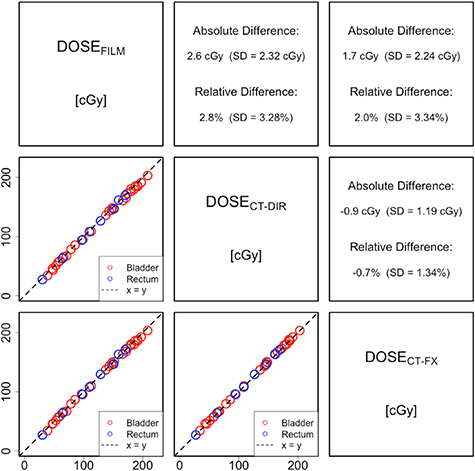
Standard image High-resolution imageAverage measured and exported 2D dose distribution values from treatment plans, as well as their absolute and relative differences are shown in figure 4. The largest difference found was between DOSEFILM and DOSECT-DIR, which amounted 2.6 cGy (2.8% relative difference). A significant albeit low, relative dose difference between DOSECT-DIR and DOSECT-FX of −0.7% (p< 0.01) also was observed. Overall, the dose calculation based on CTFX showed a slight better agreement with film measurements than the dose calculation based on CTDIR. The relative and absolute dose differences were found to be statistically significant according to the Wilcoxon signed rank test (p < 0.01). The Pearson correlation coefficient was >0.999 for all datasets comparisons.
Figure 4. Overview of average measured film readouts and exported 2D dose distribution values from treatment plans for all film measurements in the phantom. The absolute and relative differences are summarized for each pair of measurements and/or calculated values.
Download figure:
Standard image High-resolution image3.1. Gamma evaluation
Table 1 shows the average gamma values for all film positions per fraction, as well as their maximum, minimum and pass-rate values. There was a small non-significant difference between the average gamma value for bladder and rectum positions: 0.31 vs 0.28, respectively (p = 0.35). High-gamma pass-rates were found for all films and conditions (different densities inside the rectum) indicating a good agreement between the measurements and dose calculations.
Table 1. Gamma analysis results for all films in the phantom.
| Fraction | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Bladder filling [cc] | 120 | 98 | 232 | 150 |
| Rectum filling [cc] | 2 (air) | 2 (water) | 3 (water) | 2 (air) |
| Average Gamma Bladder | 0.35 | 0.21 | 0.33 | 0.36 |
| Average Gamma Rectum | 0.34 | 0.25 | 0.26 | 0.28 |
| Pass Rate Bladder | 99.3 | 100.0 | 97.8 | 96.4 |
| Pass Rate Rectum | 97.1 | 100.0 | 100.0 | 98.9 |
| Min average gamma | 0.22 | 0.18 | 0.18 | 0.24 |
| Max average gamma | 0.46 | 0.29 | 0.58 | 0.50 |
4. Discussion
Verification and safety of treatment delivery have always been a key component in the field of radiotherapy. Confidence and estimation of the accuracy of the delivered dose are necessary before clinical introduction of new techniques. We have devised an end-to-end test for MRgRT adaptive workflows in which all steps needed for patient alignment, online plan adaptation and treatment delivery are accounted for. For this purpose we used the combination of an anthropomorphic phantom and film dosimetry to simulate the delivery of several fractions for MRgRT in prostate cancer. The agreement between dose calculations and experimental film measurements was excellent with average relative dose differences <3% for both, DOSECT-DIR and DOSECT-FX. In addition, high average gamma pass-rates for all fractions were obtained.
The degree of agreement achieved with our end-to-end test for MRgRT is similar to previous reported results for IMRT/VMAT with a variety of phantoms. An inter-center quality assurance program for IMRT verification reported a maximum local deviation of 3.5% in the mean dose to the PTV and 5% in the OAR (Gillis et al 2005). Results of an end-to-end test for volumetric arc therapy showed gamma pass-rates >93% and a median absolute point dose deviation of 1.2% (O'Daniel et al 2012). Furthermore, measurements on an anthropomorphic pelvic phantom using an ionization chamber and thermoluminescent dosimeters (TLDs) reported dose differences ranging −3.3 to +1.6% (Harrison et al 2011). In this study we found a slightly better agreement with film measurements when using the electron density of a CT representing the anatomy of that particular fraction (CTFX) than using the deformed CT from baseline (CTDIR). However, the relative difference between both was small (−0.7%) and although both DOSECT-DIR and DOSECT-FX were systematically lower than DOSEFILM it cannot be completely ruled out that this may be a film calibration effect. In the past, significant errors in film measurements induced by the presence of a magnetic field have been reported (Delfs et al 2018), and although this cannot be fully discarded, in our end-to-end test the film calibration and measurements were both made in the presence of the magnetic field to minimize this potential effect as much as possible. No significant dependency of average gamma or pass rate on film position was found.
Several distinct features made our end-to-end test especially valuable, namely (1) the use of an anthropomorphic phantom with tissue equivalent materials and different electron densities for the region of interest comprised by the rectum and bladder; (2) the ability to measure at different locations and orientations at the surfaces of the OARs, with some of them at the interface with the PTV; (3) the dose recording in low- and high-dose areas and in steep dose gradient regions; and (4) the simulation of a complete MRgRT adaptive workflow, including inter-fractional changes, deformable image registration and online re-planning. However, it is also worth noting that the described end-to-end test has some limitations. Firstly, due to the nature of the stationary phantom, in our tests there were no blurring or ghosting artifacts due to breathing or patient motion. However, in clinical practice a repeated scan is taken when ghosting artifacts due to patient motion are observed. Intra-fractional anatomical changes such as rectal motion or increased bladder filling during the relatively long treatment times are not simulated in the described setup. Therefore, the obtained measurement results provide an estimation of the accuracy of the dose as calculated and delivered in a stationary situation. It is however worth mentioning, that in the clinical workflow, these anatomical changes are mitigated by real time 2-D imaging, on which the CTV is tracked during treatment.
Secondly, film dosimetry is a labor intensive and error-prone process. Adding an ionization chamber measurement to the experimental setup would partially overcome these limitations, and also provide a secondary check of the dosimetric accuracy of the treatment delivery. The advantage of using relatively small pieces of film is that it allowed measurements on the interface of the OARs with the target. There were no dosimetric pockets inside the target and therefore the delivered dose to the center of the PTV could not be verified. A re-design of the OARs or target such that they can be fully intersected by a film would be of great benefit.
The multimodal and anthropomorphic phantom was especially suited for the end-to-end test experimental validation in MRgRT by allowing dosimetric film measurements at relevant locations in the phantom under different conditions (OAR changes). Our measurements exhibited a very good agreement with the planned dose distribution after following an online MRgRT adaptive procedure. This yields large potential for expanding verification measurements in MRgRT and could be applied for deformable image registration (DIR) based dose accumulation, where controlled inter-fractional variations of bladder and rectum can be introduced.