Abstract
Per capita fructose consumption has increased 100-fold over the last century1. Epidemiological studies suggest that excessive fructose consumption, and especially consumption of sweet drinks, is associated with hyperlipidaemia, non-alcoholic fatty liver disease, obesity and diabetes2,3,4,5,6,7. Fructose metabolism begins with its phosphorylation by the enzyme ketohexokinase (KHK), which exists in two alternatively spliced forms8. The more active isozyme, KHK-C, is expressed most strongly in the liver, but also substantially in the small intestine9,10 where it drives dietary fructose absorption and conversion into other metabolites before fructose reaches the liver11,12,13. It is unclear whether intestinal fructose metabolism prevents or contributes to fructose-induced lipogenesis and liver pathology. Here we show that intestinal fructose catabolism mitigates fructose-induced hepatic lipogenesis. In mice, intestine-specific KHK-C deletion increases dietary fructose transit to the liver and gut microbiota and sensitizes mice to fructose’s hyperlipidaemic effects and hepatic steatosis. In contrast, intestine-specific KHK-C overexpression promotes intestinal fructose clearance and decreases fructose-induced lipogenesis. Thus, intestinal fructose clearance capacity controls the rate at which fructose can be safely ingested. Consistent with this, we show that the same amount of fructose is more strongly lipogenic when drunk than eaten, or when administered as a single gavage, as opposed to multiple doses spread over 45 min. Collectively, these data demonstrate that fructose induces lipogenesis when its dietary intake rate exceeds the intestinal clearance capacity. In the modern context of ready food availability, the resulting fructose spillover drives metabolic syndrome. Slower fructose intake, tailored to intestinal capacity, can mitigate these consequences.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (J.D.R.) upon request. Source data are provided with this paper.
References
Johnson, R. J. et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 86, 899–906 (2007).
Te Morenga, L., Mallard, S. & Mann, J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 346, e7492 (2012).
Tsilas, C. S. et al. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. CMAJ 189, E711–E720 (2017).
Chung, M. et al. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am. J. Clin. Nutr. 100, 833–849 (2014).
Chiu, S. et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 68, 416–423 (2014).
Zhang, Y. H. et al. Very high fructose intake increases serum LDL-cholesterol and total cholesterol: a meta-analysis of controlled feeding trials. J. Nutr. 143, 1391–1398 (2013).
Sievenpiper, J. L. et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann. Intern. Med. 156, 291–304 (2012).
Heinz, F., Lamprecht, W. & Kirsch, J. Enzymes of fructose metabolism in human liver. J. Clin. Invest. 47, 1826–1832 (1968).
Patel, C. et al. Effect of dietary fructose on portal and systemic serum fructose levels in rats and in KHK−/− and GLUT5−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G779–G790 (2015).
Ishimoto, T. et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl Acad. Sci. USA 109, 4320–4325 (2012).
Patel, C. et al. Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R499–R509 (2015).
Villegas, L. R. et al. Effects of fructose-containing sweeteners on fructose intestinal, hepatic, and oral bioavailability in dual-catheterized rats. PLoS ONE 13, e0207024 (2018).
Jang, C. et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 27, 351–361.e3 (2018).
Diggle, C. P. et al. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiol. Genomics 42A, 235–243 (2010).
Lowry, O. H., Carter, J., Ward, J. B. & Glaser, L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J. Biol. Chem. 246, 6511–6521 (1971).
Zhao, S. et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579, 586–591 (2020).
Smith, G. I. et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 130, 1453–1460 (2020).
Kolderup, A. & Svihus, B. Fructose metabolism and relation to atherosclerosis, type 2 diabetes, and obesity. J. Nutr. Metab. 2015, 823081 (2015).
Sun, S. Z. & Empie, M. W. Fructose metabolism in humans: what isotopic tracer studies tell us. Nutr. Metab. (Lond.) 9, 89 (2012).
Hannou, S. A., Haslam, D. E., McKeown, N. M., & Herman, M. A. Fructose metabolism and metabolic disease. J. Clin. Invest. 128, 545–555 (2018).
Softic, S., Cohen, D. E. & Kahn, C. R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 61, 1282–1293 (2016).
Mirtschink, P., Jang, C., Arany, Z. & Krek, W. Fructose metabolism, cardiometabolic risk, and the epidemic of coronary artery disease. Eur. Heart J. 39, 2497–2505 (2018).
Lyssiotis, C. A. & Cantley, L. C. Metabolic syndrome: F stands for fructose and fat. Nature 502, 181–182 (2013).
Jegatheesan, P. & De Bandt, J.-P. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients 9, 230 (2017).
Caliceti, C., Calabria, D., Roda, A. & Cicero, A. F. G. Fructose intake, serum uric acid, and cardiometabolic disorders: a critical review. Nutrients 9, 395 (2017).
Bray, G. A., Nielsen, S. J. & Popkin, B. M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 79, 537–543 (2004).
Spector, A. A. & Kim, H.-Y. Discovery of essential fatty acids. J. Lipid Res. 56, 11–21 (2015).
Ameer, F., Scandiuzzi, L., Hasnain, S., Kalbacher, H. & Zaidi, N. De novo lipogenesis in health and disease. Metabolism 63, 895–902 (2014).
Duran, M., Beemer, F. A., Bruinvis, L., Ketting, D. & Wadman, S. K. D-glyceric acidemia: an inborn error associated with fructose metabolism. Pediatr. Res. 21, 502–506 (1987).
Cross, N. C. et al. Molecular analysis of aldolase B genes in hereditary fructose intolerance. Lancet 335, 306–309 (1990).
Lanaspa, M. A. et al. Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J. Clin. Invest. 128, 2226–2238 (2018).
Kim, M. et al. Intestinal, but not hepatic, ChREBP is required for fructose tolerance. JCI Insight 2, e96703 (2017).
Kim, M.-S. et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J. Clin. Invest. 126, 4372–4386 (2016).
Sundborn, G. et al. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity (Silver Spring) 27, 879–887 (2019).
Wang, J. et al. Associations between added sugar (solid vs. liquid) intakes, diet quality, and adiposity indicators in Canadian children. Appl. Physiol. Nutr. Metab. 40, 835–841 (2015).
Goncalves, M. D. et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349 (2019).
DiMeglio, D. P. & Mattes, R. D. Liquid versus solid carbohydrate: effects on food intake and body weight. Int. J. Obes. Relat. Metab. Disord. 24, 794–800 (2000).
Togo, J., Hu, S., Li, M., Niu, C. & Speakman, J. R. Impact of dietary sucrose on adiposity and glucose homeostasis in C57BL/6J mice depends on mode of ingestion: liquid or solid. Mol. Metab. 27, 22–32 (2019).
Dreher, M. L. Whole fruits and fruit fiber emerging health effects. Nutrients 10, 1833 (2018).
Hebden, L. et al. Fruit consumption and adiposity status in adults: a systematic review of current evidence. Crit. Rev. Food Sci. Nutr. 57, 2526–2540 (2017).
Guyenet, S. J. Impact of whole, fresh fruit consumption on energy intake and adiposity: a systematic review. Front. Nutr. 6, 66 (2019).
Keast, D. R., O’Neil, C. E. & Jones, J. M. Dried fruit consumption is associated with improved diet quality and reduced obesity in US adults: National Health and Nutrition Examination Survey, 1999–2004. Nutr. Res. 31, 460–467 (2011).
Sharma, S. P., Chung, H. J., Kim, H. J. & Hong, S. T. Paradoxical effects of fruit on obesity. Nutrients 8, 633 (2016).
Zheng, M. et al. Liquid versus solid energy intake in relation to body composition among Australian children. J. Hum. Nutr. Diet. 28, 70–79 (2015).
Wang, J. et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J. Nutr. 144, 81–86 (2014).
Malik, V. S. & Hu, F. B. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J. Am. Coll. Cardiol. 66, 1615–1624 (2015).
Zou, Z.-Y., Shen, B. & Fan, J.-G. Systematic review with meta-analysis: epidemiology of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 25, 1764–1772 (2019).
Chao, C.-Y. et al. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: a review article. World J. Gastroenterol. 22, 7727–7734 (2016).
Sourianarayanane, A. et al. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J. Crohns Colitis 7, e279–e285 (2013).
Miller, C. O. et al. Ketohexokinase knockout mice, a model for essential fructosuria, exhibit altered fructose metabolism and are protected from diet-induced metabolic defects. Am. J. Physiol. Endocrinol. Metab. 315, E386–E393 (2018).
Calle, R., Bergman, A., Somayaji, V., Chidsey, K. & Kazierad, D. PS-110-Ketohexokinase inhibitor PF-06835919 administered for 6 weeks reduces whole liver fat as measured by magnetic resonance imaging-proton density fat fraction in subjects with non-alcoholic fatty liver disease. J. Hepatol. 70, E69–E70 (2019).
Huard, K. et al. Discovery of fragment-derived small molecules for in vivo inhibition of ketohexokinase (KHK). J. Med. Chem. 60, 7835–7849 (2017).
Su, X., Lu, W. & RabinowitzJ. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Zhang, Z., Chen, L., Liu, L., Su, X. & Rabinowitz, J. D. Chemical basis for deuterium labeling of fat and NADPH. J. Am. Chem. Soc. 139, 14368–14371 (2017).
Acknowledgements
This work was supported by a grant from the DRC Regional Metabolomics Core (no. P30 DK19525 to J.D.R.), National Institutes of Health Pioneer grant (no. 1DP1DK113643 to J.D.R.) and grant no. DK107667 to Z.A. C.J. was a postdoctoral fellow of the American Diabetes Association (no. 1–17-PDF-076). We thank members of the Arany and Rabinowitz laboratories for scientific discussions.
Author information
Authors and Affiliations
Contributions
C.J., S.W., Z.A. and J.D.R. designed the study. C.J. and S.W. performed most of the experiments. S.W. generated the intestine-specific KHK-C transgenic mice. C.J. and S.Y. generated the intestine-specific KHK-C knockout mice. S.Y. and B.G. contributed to sample preparation, gene expression studies, organ histology and genotyping. X.Z. contributed to the isotope tracing studies. Z.Z. and Y.S. helped with the lipogenesis calculations. G.L. performed the qRT–PCR analysis. C.J., S.W., Z.A. and J.D.R. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.D.R. is a consultant and receives research funding from Pfizer and is an advisor of Colorado Research Partners and cofounder of VL54. All other authors declare no conflicts of interest.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
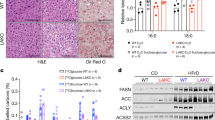
Extended Data Fig. 1 Intestine-specific Khk-C ablation increases fructose spillover to the colonic microbiota.
a, Khk-C floxed mice were generated by inserting two loxP sequences (black arrowheads) on both sides of a Khk-C specific exon (orange). The mice were then crossed with the Villin-Cre mice to generate intestine-specific Khk-C KO mice. b, qPCR (left) and western blots (right) show ablation of Khk-C in the jejunum but not in the liver (N = 6 mice for WT, 4 mice for KO). c, Khk-A mRNA is induced in the jejunum of KO mice (N = 6 mice for WT, 4 mice for KO). d, e, Mice received 1:1 mixture of 13C-fructose and unlabeled glucose (1 g/kg each) via oral gavage and the labeled F1P in duodenum and jejunum were measured in d and e, respectively (N = 3 mice for each time point). The AUC is shown on the right for the jejunum in e. f, AUC of labeled fructose in the cecal content over 1 h after gavage (N = 3 mice per group). g, Concentrations of total labeled carbons in acetate, propionate and butyrate in the cecal content 1 h or 2 h after gavage (N = 4, 3, 5, 5 mice). Data are mean ± standard error. Numbers in graphs indicate P-values by two-sided Student’s t-test.
Extended Data Fig. 2 Metabolic characterization of intestine-specific Khk-C KO mice.
Mice received normal water or 10% sucrose water for 8 weeks. a, Fructolytic gene expression in the jejunum (N = 4, 6, 7, 12 mice). b, Metabolic parameters. P-values were calculated by two-sided Student’s t-test without adjustment for multiple comparisons. c, Body weights (N = 5 mice per group, except N = 4 mice for male KO). d, Epididymal fat pad weights (N = 5 mice per group, except N = 4 mice for male KO). Data are mean ± standard error.
Extended Data Fig. 3 Intestine-specific Khk-C ablation increases hepatic lipogenesis.
a, Mice received 1:1 mixture of fructose and glucose (either hexose 13C-labeled, 2 g/kg each) via oral gavage. After 6 h, the labeling of saponified palmitate in serum, liver and jejunum was compared (N = 6 mice per group) IC, Ion count. b, Mice received normal water or 10% sucrose water for 8 weeks. Mice received 50% D2O in the drinking water overnight. The next morning, circulating fatty acid labeling was measured. Data are reported as the total number of deuterium atoms assimilated into the indicated saponified fatty acid (product of fatty acid concentration x average number of deuteriums per fatty acid molecule) (N = 7, 8, 4, 7 mice). c, d, Fractional contribution of fructose carbons into lipogenic acetyl-CoA pools. The acetyl group labeling is inferred based on the labeled palmitate mass isotopic distribution measured 6 h after 13C-fructose gavage (with unlabeled glucose, 2 g/kg each) in c (N = 6, 6, 8, 9 mice), or 24 h after ad lib drinking fructose and glucose mixture (15% each, either hexose 13C-labeled) in d (N = 4, 5, 4, 5 mice). Data are mean ± standard error. Numbers in graphs indicate P-values by two-sided Student’s t-test.
Extended Data Fig. 4 Intestine-specific Khk-C ablation worsens hyperlipidemia after chronic sucrose drinking.
Mice received 10% sucrose water for 1 month or 3 months. Heatmap shows circulating lipid profiles measured by LC-MS. Each lipid species was normalized by the average of all mice (N = 6, 3, 8, 6 mice). TG, triglyceride; DG, diglyceride.
Extended Data Fig. 5 Intestine-specific Khk-C ablation worsens hepatic steatosis after chronic sucrose drinking.
Mice received normal water (NW) or 10% sucrose water (Suc) for 5 months. a, Heatmap shows hepatic lipid profiles measured by LC-MS. Each lipid species was normalized by the average of all mice (N = 4, 5, 8, 7 mice). b, Sucrose drinking increases saturated triglycerides, consistent with increased lipogenesis. Note that the trend is stronger in the intestine-specific Khk-C KO mice. Y-axis is a log scale. Numbers on the x-axis (for example, 48:1) mean the total number of carbons (for example, 48) and the number of double bonds (for example, 1) in triglyceride, with more unsaturated triglycerides having more double bonds (N = 4, 5, 8, 7 mice). Data are mean ± standard error.
Extended Data Fig. 6 Hepatic gene expression profiles of intestine-specific Khk-C KO mice after chronic sucrose drinking.
a–c, Mice received normal water or 10% sucrose water for 5 months. Then, the livers were isolated at 9 AM and the mRNA levels of the indicated genes were measured (N = 6, 6, 11, 15 mice). Data are mean ± standard error. Numbers in graphs indicate P-values by two-sided Student’s t-test.
Extended Data Fig. 7 Intestine-specific Khk-C overexpression increases intestinal fructose catabolism.
a, Khk-C inducible overexpressing transgenic (TG) mice were generated by cloning a Khk-C coding sequence (orange) under the TET-on promoter (green). Then, the mice were crossed with mice that harbored Villin-Cre driver (blue) and rtTA with a stop-codon (gray) in the ROSA26 genomic region. Mice were fed with doxycycline in the drinking water to induce deletion of the stop-codon and subsequent Khk-C overexpression in the Villin-expressing intestinal epithelial cells. b, qPCR (left) and western blots (right) show Khk-C induction in the jejunum but not in the liver (N = 4 mice per group). The exposure time for Khk-C blot in the jejunum was decreased to prevent signal saturation. c, Khk-A mRNA levels were measured in the liver or jejunum (N = 4 mice per group). d, e, Mice received 1:1 mixture of 13C-fructose and unlabeled glucose (1 g/kg each) via oral gavage and the levels of labeled F1P and glycerate in the jejunum were measured in d, e, respectively (N = 3 mice for each time point). AUC is shown on the right. Data are mean ± standard error. Numbers in graphs indicate P-values by two-sided Student’s t-test.
Extended Data Fig. 8 Intestine-specific Khk-C overexpression induces fructose aversion.
Mice received normal water or sucrose water for 3 months. a, Body weights (N = 5, 6 mice). b, Heatmap shows circulating lipid profiles measured by LC-MS. Each lipid species was normalized by the average of all mice (N = 3, 6, 4, 5 mice). c, Daily water consumption (N = 6, 5, 4, 5 mice). Data are mean ± standard error. Number in the graph indicates a P-value by two-sided Student’s t-test.
Extended Data Fig. 9 Intestine-specific Khk-C TG overexpression induces cecal fructose catabolism and reduces lipogenesis.
a–d, Mice received normal water or sucrose water for 3 months. Mice received 1:1 mixture of 13C-fructose and unlabeled glucose (1 g/kg) via oral gavage and the AUC of cecal labeled F1P was measured in a (N = 3 mice per group). Representative hematoxylin & eosin stained jejunum sections from two independent experiments are shown in b. Mice received 1:1 mixture of 13C-fructose and unlabeled glucose (1 g/kg) via oral gavage and the AUC of labeled fructose in the cecal content was measured in c (N = 4, 3 mice). The mRNA levels of fructolytic genes in the cecum was measured in d (N = 7, 6 mice). e-g, Mice received 1:1 mixture of fructose and glucose (2 g/kg each) via oral gavage twice a day for five days. The mRNA levels of fructolytic genes in the jejunum was measured in e (N = 5, 4, 5, 6 mice). On the first day (day 1) or the last day (day 5), the mice received 1:1 mixture of 13C-fructose and unlabeled glucose (2 g/kg each) via oral gavage and the 13C-labeling of the indicated circulating saponified fatty acids was measured after 6 h in f (N = 10 mice for WT, 6 mice for TG). Alternatively, on day 5, mice received 100% D2O via intra-peritoneal injection and the deuterium labeling of the indicated circulating saponified fatty acids was measured after 6 h in g (N = 5 mice per group). Data are mean ± standard error. Numbers in graphs indicate P-values by two-sided Student’s t-test.
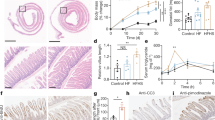
Extended Data Fig. 10 Solid form of fructose consumption suppresses fructose-induced lipogenesis.
a, Mice received 1:1 mixture of 13C-fructose and unlabeled glucose (2 g/kg) via oral gavage. The same total quantity of fructose was administered as a single high-dose or divided doses over 45 min. Then, for each group, intestinal fructose catabolism was quantitated, using the labeled metabolite concentration differences between the portal and systemic blood (N = 3 mice per group). b, Experimental design for fructose drinking (10% sucrose drinking water) or eating (10% sucrose hydrogel). Mice were provided the liquid or solid containing sucrose for 7 days. On day 7, the water (in either the sucrose drinking water or the sucrose hydrogel) was 25% D2O. This served two purposes: (i) enabling precise tracking of total consumption based on circulating D2O enrichment and (ii) lipogenesis measurement. On day 8, blood was harvested. c, d, The mice that received the sweetened hydrogel showed less lipogenesis than the mice that received the sweetened drinking water. Deuterium labeling of circulating water was indistinguishable in c (N = 9, 6 mice), indicating identical total fructose consumption. Deuterium labeling of circulating saponified fatty acids was greater in the liquid group in d (N = 9, 6 mice). Data are mean ± standard error. Numbers in graphs indicate P-values by two-sided Student’s t-test.
Supplementary information
Source data
Source Data Extended Data Fig. 1
Unprocessed western Blots for Extended Data Fig. 1b.
Source Data Extended Data Fig. 7
Unprocessed western Blots for Extended Data Fig. 7b.
Rights and permissions
About this article
Cite this article
Jang, C., Wada, S., Yang, S. et al. The small intestine shields the liver from fructose-induced steatosis. Nat Metab 2, 586–593 (2020). https://doi.org/10.1038/s42255-020-0222-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0222-9
This article is cited by
-
Are all sugars equal? Role of the food source in physiological responses to sugars with an emphasis on fruit and fruit juice
European Journal of Nutrition (2024)
-
Pharmacotherapies of NAFLD: updated opportunities based on metabolic intervention
Nutrition & Metabolism (2023)
-
Fructose promotes angiogenesis by improving vascular endothelial cell function and upregulating VEGF expression in cancer cells
Journal of Experimental & Clinical Cancer Research (2023)
-
The role of ChREBP in carbohydrate sensing and NAFLD development
Nature Reviews Endocrinology (2023)
-
Liver-derived metabolites as signaling molecules in fatty liver disease
Cellular and Molecular Life Sciences (2023)