Abstract
Cancer cells adapt their metabolic activities to support growth and proliferation. However, increased activity of metabolic enzymes is not usually considered an initiating event in the malignant process. Here, we investigate the possible role of the enzyme serine hydroxymethyltransferase-2 (SHMT2) in lymphoma initiation. SHMT2 localizes to the most frequent region of copy number gains at chromosome 12q14.1 in lymphoma. Elevated expression of SHMT2 cooperates with BCL2 in lymphoma development; loss or inhibition of SHMT2 impairs lymphoma cell survival. SHMT2 catalyzes the conversion of serine to glycine and produces an activated one-carbon unit that can be used to support S-adenosyl methionine synthesis. SHMT2 induces changes in DNA and histone methylation patterns leading to promoter silencing of previously uncharacterized mutational genes, such as SASH1 and PTPRM. Together, our findings reveal that amplification of SHMT2 in cooperation with BCL2 is sufficient in the initiation of lymphomagenesis through epigenetic tumor suppressor silencing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Sequencing (RNA-seq, proton sequencing, ChIP–seq and ERRBS) data that support the findings of this study have been deposited in the Gene Expression Omnibus under accession codes GSE142336 and GSE139523. Previously published microarray data that were reanalyzed in this study are available under accession code GSE132929. Histone mass spectrometry data were deposited to MassIVE under accession code MSV000085251. Source data for Figs. 1–7 and Extended Data Fig. 2 have been provided as Source Data Files 1–12. All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Any custom computer code or algorithm previously used to generate results that are reported in this paper and central to its main claims are available upon request.
References
Basso, K. & Dalla-Favera, R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 15, 172–184 (2015).
Huet, S., Sujobert, P. & Salles, G. From genetics to the clinic: a translational perspective on follicular lymphoma. Nat. Rev. Cancer 18, 224–239 (2018).
Lenz, G. & Staudt, L. M. Aggressive lymphomas. N. Engl. J. Med. 362, 1417–1429 (2010).
Pasqualucci, L. et al. Genetics of follicular lymphoma transformation. Cell Rep. 6, 130–140 (2014).
Haluska, F. G., Finver, S., Tsujimoto, Y. & Croce, C. M. The t(8; 14) chromosomal translocation occurring in B-cell malignancies results from mistakes in V–D–J joining. Nature. 324, 158–161 (1986).
Kridel, R., Sehn, L. H. & Gascoyne, R. D. Pathogenesis of follicular lymphoma. J. Clin. Invest. 122, 3424–3431 (2012).
Nunez, G. et al. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc. Natl Acad. Sci. USA 86, 4589–4593 (1989).
Ortega-Molina, A. et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med. 21, 1199–1208 (2015).
Jiang, Y. et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 7, 38–53 (2017).
Zhang, J. et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat. Med. 21, 1190–1198 (2015).
Zhang, J. et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B-cell lymphoma. Cancer Discov. 7, 322–337 (2017).
De, S. et al. Aberration in DNA methylation in B-cell lymphomas has a complex origin and increases with disease severity. PLoS Genet. 9, e1003137 (2013).
Kretzmer, H. et al. DNA methylome analysis in Burkitt and follicular lymphomas identifies differentially methylated regions linked to somatic mutation and transcriptional control. Nat. Genet. 47, 1316–1325 (2015).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 (2014).
Kaelin, W. G. Jr. & McKnight, S. L. Influence of metabolism on epigenetics and disease. Cell 153, 56–69 (2013).
Maddocks, O. D. K., Labuschagne, C. F., Adams, P. D. & Vousden, K. H. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol. Cell 61, 210–221 (2016).
Kim, D. et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520, 363–367 (2015).
Anderson, D. D., Quintero, C. M. & Stover, P. J. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc. Natl Acad. Sci. USA 108, 15163–15168 (2011).
Morscher, R. J. et al. Mitochondrial translation requires folate-dependent tRNA methylation. Nature 554, 128–132 (2018).
Minton, D. R. et al. Serine catabolism by SHMT2 is required for proper mitochondrial translation initiation and maintenance of formylmethionyl-tRNAs. Mol. Cell 69, 610–621.e5 (2018).
Kottakis, F. et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature 539, 390–395 (2016).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Oricchio, E. et al. Frequent disruption of the RB pathway in indolent follicular lymphoma suggests a new combination therapy. J. Exp. Med. 211, 1379–1391 (2014).
Egle, A., Harris, A. W., Bath, M. L., O’Reilly, L. & Cory, S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood 103, 2276–2283 (2004).
Pajic, A. et al. Cell cycle activation by c-myc in a Burkitt lymphoma model cell line. Int. J. Cancer 87, 787–793 (2000).
Ducker, G. S. et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 114, 11404–11409 (2017).
Ding, J. et al. The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab. 18, 896–907 (2013).
Shyh-Chang, N. et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222–226 (2013).
Stevenson, W. S. et al. DNA methylation of membrane-bound tyrosine phosphatase genes in acute lymphoblastic leukaemia. Leukemia 28, 787–793 (2014).
Shan, Z., Parker, T. & Wiest, J. S. Identifying novel homozygous deletions by microsatellite analysis and characterization of tumor suppressor candidate 1 gene, TUSC1, on chromosome 9p in human lung cancer. Oncogene 23, 6612–6620 (2004).
La Rochelle, J. et al. Chromosome 9p deletions identify an aggressive phenotype of clear cell renal cell carcinoma. Cancer 116, 4696–4702 (2010).
Caro, P. et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 22, 547–560 (2012).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Cairns, R. A. & Mak, T. W. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 3, 730–741 (2013).
Hoadley, K. A. et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304.e6 (2018).
Witkiewicz, A. K. et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 6, 6744 (2015).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Nikiforov, M. A. et al. A functional screen for Myc-responsive genes reveals serine hydroxymethyltransferase, a major source of the one-carbon unit for cell metabolism. Mol. Cell. Biol. 22, 5793–5800 (2002).
Haggerty, T. J., Zeller, K. I., Osthus, R. C., Wonsey, D. R. & Dang, C. V. A strategy for identifying transcription factor binding sites reveals two classes of genomic c-Myc target sites. Proc. Natl Acad. Sci. USA 100, 5313–5318 (2003).
Wang, S. S. et al. Polymorphisms in DNA repair and one-carbon metabolism genes and overall survival in diffuse large B-cell lymphoma and follicular lymphoma. Leukemia 23, 596–602 (2009).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Locasale, J. W. et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 (2011).
Esteller, M. et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J. Natl Cancer Inst. 92, 564–569 (2000).
Merlo, A. et al. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat. Med. 1, 686–692 (1995).
Green, M. R. et al. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat. Commun. 5, 3904 (2014).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Wendel, H.-G. et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428, 332–337 (2004).
Béguelin, W. et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013).
Mavrakis, K. J. et al. Tumorigenic activity and therapeutic inhibition of Rheb GTPase. Genes Dev. 22, 2178–2188 (2008).
Singh, K. et al. c-MYC regulates mRNA translation efficiency and start-site selection in lymphoma. J. Exp. Med. 216, 1509–1524 (2019).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Engström, P. G. et al. Systematic evaluation of spliced alignment programs for RNA-seq data. Nat. Methods 10, 1185–1191 (2013).
Garcia, B. A. et al. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat. Protoc. 2, 933–938 (2007).
MacLean, B. et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010).
Camarillo, J. M. et al. Coupling fluorescence-activated cell sorting and targeted analysis of histone modification profiles in primary human leukocytes. J. Am. Soc. Mass Spectrom. 30, 2526–2534 (2019).
Akalin, A. et al. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 13, R87 (2012).
Sanghvi, V. R. et al. The oncogenic action of NRF2 depends on de-glycation by fructosamine-3-kinase. Cell 178, 807–819.e21 (2019).
Acknowledgements
We thank R.L. Possemato (NYU) for sharing the pMXS-IRES-blast; SHMT2res and pMXS-IRES-blast; SHMT2-CD (SHMT2 K280A) plasmids, and T. Oellerich for sharing the lentiviral catalytic dead SHMT2 (SHMT2 K280A) plasmid. We thank Raze Therapeutics for sharing the SHIN1 drug. We thank K.R. Keshari, L.W.S. Finely, W. Beguelin and L. Cerchietti for helpful discussions and suggestions. We thank V. Sanghavi, K. Singh, D. Salloum and other members of Wendel laboratory for advice and reagents. Also, we thank V. Di Gialleonardo and C. Duy. We thank all members of the MSK Antitumor Assessment Core for technical assistance with the mice; the MSK Laboratory of Comparative Pathology and MSK Flow Cytometry for their support in processing biological samples; and the Weill Cornell Epigenomics Core for performing the RNA-seq, ERBBS and ChIP–seq. We acknowledge the use of the Integrated Genomics Operation Core, funded by an NCI Cancer Center Support Grant (CCSG, P30 CA08748), Cycle for Survival and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology. This research was supported by funding from the National Institutes of Health (NIH) SPORE in Soft Tissue Sarcoma (grant no. P50 CA217694 to H.-G.W.), Starr Cancer Consortium (to H.-G.W. and B.-K), Technology Development Fund (grant no. GC230724 to H.-G.W.), Starr Cancer Consortium (grant no. I10-0064 to H.-G.W.), the Lymphoma Research Foundation (grant no. GC233089 to H.-G.W.), NIH grant nos. RO1CA183876-05, RO1CA207217-03, NIH Spore P50 CA192937-03, LLS 7014-17 and LLS 1318-15 (to H.-G.W.). H.-G.W. is a Scholar of the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Contributions
S.P. contributed to the conception and design of the study, the development of its methodology, the acquisition of data, data analysis and interpretation, and the writing and revision of the manuscript. A.O.-M. contributed to the conception and design of the study, the development of its methodology, the acquisition of data, data analysis and interpretation, and the writing and revision of the manuscript. H.-Y.Y., M.J., C.Z., J.M.C., J.P.P., P.M., S.W., P.M.T., B.-K.C., N.L.K., J.G-B., W.T., E.D.S. and N.L.K. contributed to the acquisition of data (provided animals, acquired and provided patient samples, provided facilities). J.W., M.T., E.R., D.K., N.J., A.D., N.S. and G.C. contributed to the analysis and interpretation of data (for example, statistical analysis, biostatistics, computational analysis). A.D. contributed to the analysis and interpretation of histological samples. M.R.G. contributed to the analysis and interpretation of genomics data (statistical studies) and reviewed the manuscript. S.L. contributed to the statistical analysis of data and reviewed the manuscript. K.B. contributed to the conception and design of the experiments, data analysis and interpretation, and reviewed the manuscript. A.M.M. contributed to the conception and design of the study, the development of its methodology, data analysis and interpretation, and reviewed the manuscript. H.-G.W. contributed to the conception and design of the study, the development of its methodology, data analysis and interpretation, and wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.D. has received personal consultancy fees from Roche, Corvus Pharmaceuticals, Physicians’ Education Resource, Seattle Genetics, Takeda, EUSA Pharma and AbbVie, and research grants from Roche. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
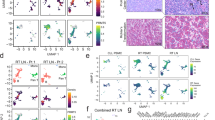
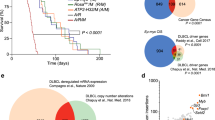
Extended Data Fig. 1 De novo serine synthesis pathway in human B cell lymphoma.
a, Bar graphs presenting the functional gain (red) or loss (blue) of SHMT2 located on chromosome 12 by DNA copy number analysis of 568 DLBCL and 176 FL tumors. b, The frequency of loss (blue), gain (red) or diploid (black) status of serine synthesis pathway genes in 568 human DLBCL and 176 human FL tumors. c, Diagrams demonstrating the overlaps of amplification (red) and loss (blue) of SHMT2 vs other serine biosynthesis pathway enzymes (PSPH, PHGDH, PSAT1, SHMT1) in 568 DLBCL and 176 FL tumors. d, Bar graph showing the frequency of SHMT2 amplification in different subtype (GC-like, ABC-like or unclassified) of 249 human DLBCL tumors.
Extended Data Fig. 2 SHMT2 promotes lymphomagenesis in vivo.
a, Diagram of FL mouse model. Fetal VavPBcl2 HSCs were transduced by MSCV-GFP plasmid carrying SHMT2 cDNA or empty vector and injected to lethally irradiated female mice. b, Representative graphs of flow cytometry analysis comparing GFP+ HSCs before injection vs GFP+ splenic lymphoma cells from VavPBcl2;vector- and VavPBcl2;SHMT2- induced tumors collected 5 months after injection. c, Dot plot representing the initial GFP+ cells in hematopoietic stem cells before injection vs GFP+ cells enriched in splenic cells collected from VavP-Bcl2;vector (N = 5 mice) and VavP-Bcl2;SHMT2 (N = 10 mice) tumors. Two-tailed Student’s t-test was used to determine statistical significance; VavP-Bcl2;vector: P(HSCvsLymphoma)= 0.443, NS; VavP-Bcl2;SHMT2: P(HSCvsLymphoma)=0.0006. d, Representative images of histology studies of VavPBcl2;vector and VavPBcl2;SHMT2 lung. The slides were stained with H&E, and antibodies for B220, TUNEL, Ki67, PNA. This experiment was independently repeated three times with similar results. Scale Bars, 500 nm. e, tumor clonality analysis on B220+ cDNA collected from VavPBcl2;vector vs VavPBcl2;SHMT2 tumors. Each lane corresponds to one tumor. This experiment was independently repeated two times with four independent samples in each genotype with similar results f, Immunoblot against SHMT2, SHMT1 and ACTIN in DLBCL cell lines carrying two different short hairpins against SHMT2. This experiment was independently repeated two times with similar results. The uncropped images of the original blots are presented in Source Data Extended Data File 12. The numerical data for this figure are presented in Source Data Extended Data File 2.
Supplementary information
Supplementary Tables
Supplementary Tables 1–13.
Source data
Source Data Fig. 1
Numerical data for Fig. 1.
Source Data Fig. 2
Unprocessed western blots for Fig. 2b.
Source Data Fig. 2
Numerical data for Fig. 2.
Source Data Fig. 2
Histological micrographs and flow cytometry analysis of replicates in Fig. 2e,f.
Source Data Fig. 3
Unprocessed western blots for Fig. 3j.
Source Data Fig. 3
Numerical data for Fig. 3.
Source Data Fig. 4
Unprocessed western blots for Fig. 4g.
Source Data Fig. 4
Numerical data for Fig. 4.
Source Data Fig. 4
Flow cytometry analysis of replicates in Fig. 4b,f.
Source Data Fig. 5
Numerical data for Fig. 5.
Source Data Fig. 5
Unprocessed dot blots for Fig. 5.
Source Data Fig. 6
Numerical data for Fig. 6.
Source Data Fig. 7
Histological micrographs and flow cytometry analysis of replicates in Fig. 7.
Source Data Fig. 7
Numerical data for Fig. 7.
Source Data Extended Data Fig. 2
Unprocessed western blots for Extended Data Fig. 2.
Source Data Extended Data Fig. 2
Numerical data for Extended Data Fig. 2.
Rights and permissions
About this article
Cite this article
Parsa, S., Ortega-Molina, A., Ying, HY. et al. The serine hydroxymethyltransferase-2 (SHMT2) initiates lymphoma development through epigenetic tumor suppressor silencing. Nat Cancer 1, 653–664 (2020). https://doi.org/10.1038/s43018-020-0080-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-0080-0
This article is cited by
-
SHMT2 promotes papillary thyroid cancer metastasis through epigenetic activation of AKT signaling
Cell Death & Disease (2024)
-
Amino acid metabolism in tumor biology and therapy
Cell Death & Disease (2024)
-
Construction and validation of a folate metabolism-related gene signature for predicting prognosis in HNSCC
Journal of Cancer Research and Clinical Oncology (2024)
-
SHMT2 regulates esophageal cancer cell progression and immune Escape by mediating m6A modification of c-myc
Cell & Bioscience (2023)
-
The role of SHMT2 in modulating lipid metabolism in hepatocytes via glycine-mediated mTOR activation
Amino Acids (2022)