Abstract
Novel coronavirus disease (COVID-19) has attracted much attention around the world due to its rapid transmission among humans and relatively high mortality rate. Studies are increasing to find the best therapeutic approach for the disease and its management. Regenerative medicine offers various cell-tissue therapeutics and related products, such as stem cell therapy, natural killer (NK) cell therapy, Chimeric antigen receptor (CAR) T cell therapy, exosomes, and tissue products. Interestingly, mesenchymal stem cells (MSCs) can reduce inflammatory symptoms and protect against cytokine storm, which critically contributes to the COVID-19 progression. Notably, having the potentials to exert cytotoxic effects on infected cells and induce interferon production probably make NK cells a candidate for COVID-19 cell therapy. Besides, exosomes are one of the crucial products of cells that can exert therapeutic effects through the induction of immune responses and neutralizing antibody titers. The paper aims to briefly consider current options for COVID-19 therapy to show that there is no specific cure for COVID-19, and then assess the real opportunities and range of promises regenerative medicine can provide for specific treatment of COVID-19.

Therapeutic Potential of Regenerative Medicine against COVID19.
Similar content being viewed by others
Introduction
Coronaviruses are enveloped non-segmented positive-sense RNA viruses belonging to the family Coronaviridae that can infect many hosts like humans and other mammals [1]. During the past two decades, human coronaviruses (hCoVs) have caused three outbreaks; severe acute respiratory syndrome (SARS-CoV) in 2002, the Middle Eastern coronavirus respiratory syndrome (MERS-CoV), and the novel coronavirus disease in 2019 (COVID-19) [2, 3]. Compared with the two earlier outbreaks, the ongoing outbreak of COVID-19 pneumonia is more contagious, with more than six million people affected worldwide by the end of May [4].
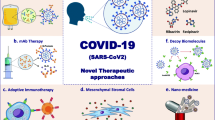
Figure 1 provides an overview of therapeutic approaches currently used for COVID-19. Among anti-viral drugs, Remdesivir, an anti-Ebola drug, seems to be most promising. There are numerous drug options based on data from the experience of treating other diseases (Fig. 2) [5]. However, each alone has insufficient efficacy in the treatment of patients with COVID-19, especially severe cases. Drug combination regimens are used to maximize their effectiveness [6], which inevitably bring side effects. Thus, there is a need for therapeutic options to control the COVID-19 outbreak while keeping side effects at a minimum. Such options include plasma therapy, monoclonal antibodies, small molecule drug-based therapies, and immunotherapies.
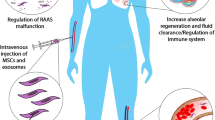
Regenerative medicine offers various cell-tissue therapeutics and related products. It deals with the use of cells themselves either as therapeutic agents or as a vehicle for other therapeutic agents such as cytokines. Of particular interest to the subject are mesenchymal stem cells that not only possess the potential for regenerative medicine but also have shown promising results in the modulation of inflammatory responses [7]. Besides, MSCs can secrete exosomes, extracellular membrane vesicles with size at the nanoscale [8]. Cell-derived exosomes serve as vectors of cell therapy acted on intercellular interactions by a range of macromolecules they can carry. Notably, exosomes have been of high importance to immune regulation and for this may become of use for cancer therapy [9, 10]. Natural killer (NK) cell therapy has also shown to produce a marked anti-tumor effect, and this effect is mostly attributed to the direct action of NK cells on the immune system [11]. In this manner, regenerative medicine and its immunoregulatory effects can be applied to the COVID-19, a viral infection associated with immune dysregulation [12].
The present study aims to briefly consider current options for COVID-19 therapy to show that there is no specific cure for COVID-19, and then assess the real opportunities and range of promises regenerative medicine can provide for specific treatment of COVID-19.
Current Evidence-Based Recommendations for COVID-19 Treatment
Plasma Therapy
The plasma of patients recovered from viral infections can be considered as an appropriate treatment option without serious adverse effects [13]. As mentioned earlier, the present century has witnessed two other pandemics caused by coronaviruses: SARS in 2002–2003 and the MERS in 2012. For both cases, convalescent plasma was a therapeutic option [14]. Although convalescent plasma therapy has not been used in the United States in decades due to the availability of better therapeutic solutions such as vaccines, it is than 100 years old. Researchers claimed that during the 1918 influenza epidemic in Spain, the transfer of survivors’ blood products, resulted in a 50% reduction in the mortality rate of critically ill patients [15]. An equivalent policy was also utilized to treat measles and polio decades ago. However, vaccine science and anti-viral drugs were developed, and plasma therapy was banned for these conditions in the 1950s [16]. The use of plasma therapy has been recommended during recent infectious outbreaks caused by Ebola [17] and H1N1 [18]. Notably, studies demonstrate that convalescent plasma is a successful therapy in reducing the mortality rate and shortening the period of hospitalization in patients with SARS-CoV and MERS-CoV [19, 20].
FDA has already announced plasma therapy as a therapeutic option for patients with COVID-19 [21]. However, this method requires gathering plasma or serum from a sufficient number of people recovered from COVID-19. Therefore, plasma therapy can be considered as an option in the treatment of patients with severe COVID-19 until a superior treatment is found [14]. Plasma therapy by passive transfer of antibodies acts on the neutralization of the virus.
In the case of inaccessibility of preparing the plasma from recovered people, monoclonal antibodies (mAbs) and genetically engineered animal hosts producing human antibodies can be considered as alternative sources of antibody preparation for SARS-CoV-2 [22]. Monoclonal antibodies have exhibited their efficacy for the treatment of SARS-CoV and MERS-CoV [23]. They, therefore, might possess the potential for the treatment of COVID-19. Noteworthy is the fact that the primary concept of passive antibody therapy is prevention and prophylaxis, while its therapeutic aspect stands on the second seat. The antibody transfer has the highest efficacy if will be administered at the early stage of the disease.
Monoclonal Antibodies
Monoclonal antibody (mAb) therapy can be used to treat many diseases, especially viral infections such as Ebola, influenza A, and HIV-1. The elderly patients are more likely to develop severe COVID-19, and their ICU admission ratio is substantially higher than that for the younger patients. Patients admitted to the ICU have higher amounts of cytokines such as IL-6, G-CSF, IP10, MCP-1, MIP1A, and TNF-α [13] and such a cytokine storm can lead to severe organ damage and death. Corticosteroids are commonly used among critically ill patients. These drugs cannot specifically target the aforementioned inflammatory cytokines, but result in a general inhibitory effect over the immune system. The use of corticosteroids may, therefore, be associated with unwanted side effects in COVID-19 [24]. Current evidence highlights the role of IL-6 in the pathogenesis of COVID-19 pneumonia. Consistently, patients with COVID-19, especially those who have high IL-6 levels, have shown clinical improvement following treatment with Tocilizumab, a monoclonal antibody that targets the IL-6 pathway [25].
Small Molecule Drug-Based Therapies
Although the novel coronavirus, SARS-CoV2, has recently been identified, the similarity of this virus with SARS-CoV in the genetic content might help the development of therapies of the novel coronavirus using previous treatments. Also, both SARS-CoV and SARS-CoV2 utilize the same host cell surface receptor. Therefore, blocking strategies used to prevent SARS-CoV cell entry might be useful against SARS-CoV2 [23]. Drugs designated based on the viral genome offer another potential therapeutic approach [26]. A salient example of these types of drugs is siRNA, small molecule drugs that can inhibit the viral replication cycle, host cell enzyme, or viral entry mechanism. Although these drugs have shown anti-viral activity in vitro and in vivo, most of them have the challenge of suppressing the immune system, and therefore, they should be used with caution.
Immune Regulation Therapy and Interferon Utilizing
Immunotherapeutic approaches have been extensively used in the treatment of cancer [27, 28], autoimmune diseases [29, 30], and other immune system-related diseases [31, 32]. They can be adopted to ameliorate immune responses in COVID-19. By considering the success of interferons in viral infections, there are ongoing studies that investigate the efficacy ofinterferon-alpha-1b and alpha-2b for COVID-19 treatment [33, 34]. Also, studies demonstrate that SARS-CoV-2 profoundly affects T cell function [33]. Patients with a severe form of the disease are most likely to possess a low number of lymphocytes, monocytes, eosinophils, and basophils and a high number of leukocytes along with a high neutrophil-lymphocyte-ratio (NLR). Both helper T (Th) cell counts and suppressor T cell counts are decreased in COVID-19 patients, and Th cell counts are much lower in the severe group of patients. Also, we witness rising the number of naïve Th cells besides falling the number of memory Th cells in severe cases. This evidence remarks on the potential of T cell-based immunotherapies for COVID-19.
The Real Opportunities for Regenerative Medicine in COVID-19 Treatment
The primary regenerative cells in the lung are stem cells and progenitor cells that maintain the stable state and repair damage to the epithelial cells of the lung [35]. Stem cells offer a potential method to treat extremely virulent coronavirus. Mesenchymal stem cells (MSCs) have shown the capacity to suppress lung inflammatory responses in the animal model of the influenza virus [36]. Recently, Chinese researchers started a clinical trial to investigate the safety and efficacy of MSCs in improving the pulmonary complications of the new coronavirus.
As of April 6, 2020, China’s clinical trial registry has included a total of 1140 cases of COVID-19, and ClinicalTrials.gov has registered 239 cases of COVID-19 (Table 1). Of these, 38 cases are planned to undergo cell therapy, either receiving stem cells (MSCs, human umbilical cord MSC, Wharton’s Jelly-MSCs, human menstrual blood-derived stem cells, and dental pulp MSCs) or NK cells (NKG2D-ACE2 CAR-NK Cells, cord blood NK Cells). None of the clinical trials registered have been completed.
During the 1980s, stem cell research emerged but remains somewhat controversial. Scientists initially considered human embryos as the source of the cells, but the theory drew fire for ethical reasons [37]. Stem cells are cells with abilities of self-renewal and multi-directional differentiation. They are highly resilient early-stage cells that can differentiate into various types of fully functioning cells leading to the generation of tissue, organ, or body fluids [38, 39]. Stem cells could be used in the treatment of various organ-specific diseases.
MSCs
MSCs exert anti-inflammatory and immune-regulatory effects, such as inhibition of atypical activation of T-lymphocytes and induction of macrophages to differentiate into anti-inflammatory macrophages and regulatory T cell subsets [40]. Also, MSCs can reduce the concentrations of pro-inflammatory cytokines like IL1α, TNFα, IL6, IL12, and IFNγ, and therefore limiting the formation of cytokine storm [40, 41]. Studies show the effects of MSCs in the treatment of acute lung injury and chronic lung disease [42]. A previous study found that MSCs can help to attenuate acute lung injury and ischemia-reperfusion mediated lung injury caused by lipopolysaccharide [43].
MSCs release cytokines either by paracrine secretion or direct interaction with immune cells, contributing to immunomodulation [44]. In other words, the cytokines secreted by activated leukocytes increase the immunomodulatory function of mesenchymal cells [45]. Mesenchymal cells inhibit the production of hydrogen peroxide by stimulated neutrophils, and thereby reducing the intensity of inflammatory stimulation [46].
MSCs can function to modulate both innate and adaptive immune responses [47]. The unique feature of these cells is the ability to suppress and modify immune cells. MSCs have inhibitory effects on different types of immune cells, such as T lymphocytes, B lymphocytes, NK natural killer cells, and dendritic cells [48]. MSCs further induce toll-like receptors (TLR) [49], innate immune sensors that detect the molecular signals of viruses, bacteria, and fungi to activate innate immune responses to the invading pathogens. The induced TLRs, in turn, serve to regulate MSC function [50].
MSCs can be a means of treatment in cytokine storm and lung injury associated with COVID-19 [46]. A recent study of seven patients with severe COVID-19 confirmed the clinical efficacy and safety of intravenous MSCs [51]. Patients showed an increase in lymphocyte count, a decrease in inflammatory markers and cytokines (such as CRP and TNF), and an increase in anti-inflammatory cytokine (IL-10). In this manner, treatment with intravenous MSCs was accompanied by the reversal of COVID-19-related immune abnormalities [51], which include lymphopenia [52] and inflammation.
The regenerative, pro-angiogenic, and anti-fibrotic paracrine effects of MSCs, in addition to their immunoregulatory effects, deserve attention in the context of COVID-19 [53]. MSCs can produce IL10, hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), and vascular endothelial growth factor (VEGF), which play a role in regenerating damaged lung tissue and resisting fibrosis, like diffuse alveolar damage (DAD) in SARS [54]. DAD is a histological pattern in lung disease, which is seen in ARDS. It seems that MSCs possess the ability to produce the required factors to overcome DAD and regenerate the lung [55]. Altogether, MSC transplantation controls inflammatory responses and counteract allergic reactions while encouraging tissue repair and regeneration. For this, MSCs can establish a lung microenvironment too ideal to be appreciated for the recovery from COVID-19 pneumonia [56].
NK Cells
NK cells are innate immune cells in the frontline of defense against viruses along with monocytes [57]. NK cells contribute to the clearance of cancerous or virally infected cells by recognizing damage-associated molecular patterns (DAMPs). DAMPs are endogenous danger molecules released from cancerous or infected cells and activate the innate immune system by interacting with pattern recognition receptors (PRRs).
When NK cells are transferred, inhibiting and activating receptors involved in different signal transduction pathways are induced to mediate the effector functions of NK cells [58]. Cytotoxic effects are the most important part of NK cell character. The affinity of NK cells to other cells is determined by the degree to which cells express the major histocompatibility complex (MHC). A lower expression of MHC molecules by infected cells and transformed malignant cells direct the transferred NK cells and their cytotoxic effects to these cells, while a regular expression of MHC does not permit normal cell killing by NK cells. Cytokines seem to be crucial for the proper functioning of NK cells. On the one hand, numerous cytokines have shown to induce NK cells, and on the other hand, NK cells can produce cytokines. Notably, NK cells can activate interferon production, which is a marker for effective anti-viral immunity [59].
Accordingly, NK cell therapy is being tested as a treatment for multiple liquid and solid tumors and also has the proven potential to treat viral infections [60]. For patients who have a defect in innate immune response, NK cell therapy is an appropriate technique for amplification of the immune system’s function. Placenta-derived NK cells are well tolerated, relatively healthy, and flexible, facilitating future applications across a variety of organs and tissues [61]. NK Cells, in combination with standard therapy, might help the improvement of clinical symptoms in patients with pneumonia. Further studies are necessary to evaluate the efficacy of NK Cells in the treatment of COVID-19 [62].
Exosomes
Exosomes are membrane-bound vesicles (EVs) that are created in the endosomal compartment. They play vital roles in cell-cell communication [63]. MSC-derived exosomes (MSCs-Ex) are key therapeutic effectors of MSCs which promote tissue regeneration and, therefore, could be a suitable alternative in cell therapy. Also, they can be classified as non-immunogenic due to a limited amount of antigenic components [64]. In recent years, various approaches have been used to deliver exosomes to target tissues in different disease models, and also, there have been some clinical trials [65]. Exosomes include more than 150 miRNA [66] and 850 proteins [67], which play roles in both pathological and physiological events. Those derived from MSCs consist of different cytokines and growth factors, such as IL-6, IL-10, TGFβ1, and HGF, which comply with the regulation of immune responses [68].
MSCs-Ex has therapeutic effects in different conditions, including cardiovascular diseases, neurological diseases, kidney diseases, and wound healing [65]. MSC-EVs-based therapy could inhibit influenza virus replication and virus-induced apoptosis in lung epithelial cells [69]. Also, they could significantly attenuate the increased airway hyper-reactivity (AHR) and pulmonary inflammation [70]. Many others have also confirmed the potential of EVs to treat lung injury and inflammation in a rapid, effective, and clinically safe manner [71].
EVs might help the treatment of acute respiratory syndrome caused by coronaviruses. Seraphin K. et al. [72] showed that exosome-based vaccines containing the Spike (S) proteins of SARS-CoV could induce high levels of neutralizing antibodies. The S glycoprotein is one of four structural proteins of SARS-CoV that mediates viral entry into the host cells [73]. Therefore, this protein could be a good target for vaccine development against SARS-CoV. The exosome vaccines contain membrane-anchored ectodomains of viral surface proteins. Therefore, the exosomes carry multiple copies of the same viral protein on their surface, which facilitates the cross-linking of the B cell receptor. Also, exosomes can include a wide range of cellular proteins such as those which support the induction of immune responses and neutralizing antibody titers [72]. Therefore, exosomes with SARS-CoV2 components might be a potential vaccine for COVID-19.
The Promise of Regenerative Medicine in COVID-19 Treatment
Recently emerged coronaviruses, SARS-CoV, MERS-CoV, and SARS-CoV2, can lead to potentially life-threatening respiratory tract infections in humans. It is mandatory to understand the mechanisms of lung injury to prevent or treat a severe form of COVID-19. Several models have been developed to model lung cells and tissue to study disease, pathogenesis, and drug discovery. Models based on specific patient cells can help develop personalized medicine.
In Vitro Engineered Lung Cell and Tissue Modeling
Cell and tissue engineering aim to simulate the structure and function of complex organs such as lungs, liver, the heart for the purpose of organ transplantation and disease modeling [74]. The disease models could help to a more accurate understanding of disease mechanisms and also investigate the effectiveness of different drugs to find the best treatment methods. In the case of lung fibrosis models, this emerging technology firstly appeared by two-dimensional (2D) cell culture models, which can be used to study individual cells through secure imaging and gene expression profiling of cells. However, they could not mimic the 3D nature of the tissue in terms of soluble gradient cues, cell-cell and cell-matrix interactions, cell polarity, and tissue maturation. In the upper level of lung modeling, two-point-five-dimensional (2.5D) cell culture models could form 3D-like fibroblast cellular adhesions and higher-level 3D epithelial acinar structures and thereby allowing epithelial maturation.
Nevertheless, they have some limitations to provide varied cell and matrix interactions similar to those existing in the human lung. Therefore, 3D cell and tissue culture models were introduced. These models mimic lung tissue microenvironment much more accurate than 2D and 2.5D models. Collagen hydrogels, acellular fibroblast-derived and lung matrices, lung spheroids and organoids, precision-cut lung slices (PCLTs), and fibrosis-on-chip are 3D in vitro tissue models for replication of lung structure, function, cell, and matrix interactions in different levels and various ranges of culture periods and high-throughput capacities intended for drug discovery of lung fibrosis [75].
Personalized Medicine and Infected Lung Modeling
There is heterogeneity among patients with the same disease in terms of clinical manifestations and therapeutic responses. Induced pluripotent stem cells (iPSCs) are a type of stem cells, which can be generated directly from a somatic cell and are being used for preparing individual disease models in order to promote patient-specific studies in personalized medicine [76]. A successful effort to generate a personalized lung disease model was recently reported [77]. This model was obtained by adhesion of iPSCs on alginate bead surface functionalized by collagen I and poly (dopamine) coated in a bioreactor system. Then, lung organoid formation from each bead was placed in an individual well of a tissue culture plate. Organoids could form in 24 h up to 7 days based on how many types of cells are included.
Viruses in the human lung environment are different from those grown in cultured cells. M. Porotto et al. (2019) modeled the human parainfluenza viruses (HPIV) type 3-infected lungs in the form of organoids [78]. This model could provide a framework for research on lung infections caused by different types of pathogens.
Current Anti-Viral Screening Models for Coronaviruses
High-throughput screening of functional drugs is critical in anti-viral drug development. So far, several cell-based cultures have been used for drug discovery of coronaviruses. For instance, cell-based assays using an infectious virus (BSL-3) and surrogate assays (BSL-2) have been used to investigate different inhibitors and small chemical compounds that inhibit the entry of the virus [79]. Moreover, MucilAir™ is a low passage human airway epithelial cell model, which shows maximum similarity with physiological conditions [80]. Also, SCR-1 cells that express GFP have been utilized to study graded doses of drugs for anti-SARS drug screening [81]. These cell-based models have been valuable to drug discovery for coronaviruses. However, it seems that 3D models of the infected lung that previously described could better mimic the natural structure and function of lung structures, mainly when they are obtained from iPSCs. Overall, these models would pave the way into the drug screening process of coronaviruses.
Conclusion
The virus responsible for the ongoing pandemic of COVID-19 has shown to be a professional pathogen that can easily spread person-to-person no matter who and where [82]. While the number of deaths attributed to COVID-19 is approaching 400.000, no specific treatment exists for the disease, and the current treatment methods for COVID-19 are confined to the previous therapeutic techniques for similar viruses or last generations of coronaviruses, including SARS- and MERS-CoVs. Such a situation calls attention from various medical specialties and subspecialties [83,84,85].
COVID-19 can involve multiple systems, including the central nervous system, the gastrointestinal system, and the respiratory system, and this will depend on its profound effects on the immune system [86,87,88]. Regenerative medicine offers various cell-tissue therapeutics and related products that might help the reversal of COVID-19-related immune dysregulation. In particular, the promising features of MSCs, including their regenerative properties and ability to differentiate into diverse cell lineages, have generated considerable interest among researchers whose work has offered intriguing perspectives on cell-based therapies for various diseases. The immunomodulatory effects of MSCs, which may assist in inhibiting cytokine storm and lung inflammation, are of particular interest for COVID-19 therapy [89]. Besides, the potentials of NK cells to exert cytotoxic effects on infected cells and induce interferon production probably make NK cells a candidate for COVID-19 cell therapy. Cell-derived exosomes can be used as vectors for macromolecules, which can influence immune cells and signaling pathways, and therefore, become interesting for COVID-19 therapy. Finally, iPSCs can help the development of a personalized approach to COVID-19 therapy.
Abbreviations
- SARS-CoV:
-
Severe acute respiratory syndrome coronavirus
- MERS:
-
the Middle East respiratory syndrome
- WHO:
-
World Health Organization
- 2019-nCoV:
-
2019 Novel CoronaVirus
- NK:
-
Natural Killer
- COVID-19:
-
CoronaVirus disease 2019
- mAb:
-
Monoclonal antibody
- H1N1:
-
Hemagglutinin Type 1 and Neuraminidase Type 1
- FDA:
-
Food and Drug Administration
- ARDS:
-
Acute respiratory distress syndrome
- NLR:
-
Neutrophil-lymphocyte-ratio
- Th:
-
T helper
- MSCs:
-
Mesenchymal stem cells
- DAMPs:
-
Damage-associated molecular patterns
- PRRs:
-
Pattern recognition receptors
- ICU:
-
Intensive care units
- IL-6:
-
Interleukin 6
- IL12:
-
Interleukin12
- IL1α:
-
Interleukin 1 alpha
- G-CSF:
-
Granulocyte-colony stimulating factor
- IP10:
-
inducible protein 10
- MCP-1:
-
Monocyte chemoattractant protein-1
- MIP1A:
-
Macrophage Inflammatory Proteins1A TNF-α: tumor necrosis factor-alpha
- NKG2D-ACE2 CAR-NK:
-
natural killer group 2D- Angiotensin-converting enzyme 2 Chimeric antigen receptor- Natural Killer
- CT:
-
Computed Tomography
- IFNγ:
-
Interferon-gamma
- TLRs:
-
Toll-like receptors
- LPS:
-
lipopolysaccharide
- HCoV19:
-
Human coronavirus19
- EVs:
-
extracellular vesicles
- TGFβ1:
-
transforming growth factor β
- HGF:
-
hepatocyte growth factor
- AHR:
-
airway hyper-reactivity
- DC-SIGN:
-
Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin
- ACE2:
-
Angiotensin-Converting Enzyme 2 Gene
- 3D:
-
Three-Dimensional
- PCLTs:
-
Precision-Cut Lung Slices
- iPSCs:
-
Induced Pluripotent Stem Cells
- HPIV:
-
Human Parainfluenza Virus
- BSL:
-
Biosafety Level
References
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., Xiao, Y., Gao, H., Guo, L., Xie, J., Wang, G., Jiang, R., Gao, Z., Jin, Q., Wang, J., & Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506.
Meo, S., Alhowikan, A. M., al-Khlaiwi, T., Meo, I. M., Halepoto, D. M., Iqbal, M., Usmani, A. M., Hajjar, W., & Ahmed, N. (2020). Novel coronavirus 2019-nCoV: Prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. European Review for Medical and Pharmacological Sciences, 24, 2012–2019.
Agostini, M. L., et al. (2018). Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio, 9(2), e00221–e00218.
https://www.worldometers.info/coronavirus/.
Jiang, X., Luo, M., Zou, Z., Wang, X., Chen, C., & Qiu, J. (2020). Asymptomatic SARS-CoV-2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. Journal of Medical Virology. https://doi.org/10.1002/jmv.25941.
Wang, Y., Fan, G., Salam, A., Horby, P., Hayden, F. G., Chen, C., Pan, J., Zheng, J., Lu, B., Guo, L., Wang, C., & Cao, B. (2019). Comparative effectiveness of combined Favipiravir and Oseltamivir therapy versus Oseltamivir Monotherapy in critically ill patients with influenza virus infection. The Journal of Infectious Diseases, 221(10), 1688–1698. https://doi.org/10.1093/infdis/jiz656.
Kode, J. A., Mukherjee, S., Joglekar, M. V., & Hardikar, A. A. (2009). Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy, 11(4), 377–391.
Yu, B., Zhang, X., & Li, X. (2014). Exosomes derived from mesenchymal stem cells. International Journal of Molecular Sciences, 15(3), 4142–4157.
Greening, D. W., Gopal, S. K., Xu, R., Simpson, R. J., & Chen, W. (2015). Exosomes and their roles in immune regulation and cancer. Seminars in Cell and Developmental Biology, 40, 72–81 Elsevier.
Li, X. B., Zhang, Z. R., Schluesener, H. J., & Xu, S. Q. (2006). Role of exosomes in immune regulation. Journal of Cellular and Molecular Medicine, 10(2), 364–375.
Yoon, S. R., Kim, T.-D., & Choi, I. (2015). Understanding of molecular mechanisms in natural killer cell therapy. Experimental & Molecular Medicine, 47(2), e141.
Saghazadeh, A., & Rezaei, N. (2020). Immune-epidemiological parameters of the novel coronavirus–a perspective. Expert Review of Clinical Immunology, 16(5), 1–6.
Xu, X., Chen, P., Wang, J., Feng, J., Zhou, H., Li, X., Zhong, W., & Hao, P. (2020). Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China Life Sciences, 63(3), 457–460.
Casadevall, A., & Pirofski, L.-A. (2020). The convalescent sera option for containing COVID-19. The Journal of Clinical Investigation, 130(4), 1545–1548.
McGuire, L. W., & Redden, W. R. (1918). The use of convalescent human serum in influenza pneumonia-a preliminary report. American Journal of Public Health (New York), 8(10), 741–744.
Greenwood, B. (2014). The contribution of vaccination to global health: past, present and future. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 369(1645), 20130433–20130433.
Services, T. (2014). Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion , as an empirical treatment during outbreaks. Interim Guidance for National Health Authorities and Blood Transfusion Services (September), pp. 1–19.
Hung, I. F. N., To, K. K. W., Lee, C. K., Lee, K. L., Chan, K., Yan, W. W., Liu, R., Watt, C. L., Chan, W. M., Lai, K. Y., Koo, C. K., Buckley, T., Chow, F. L., Wong, K. K., Chan, H. S., Ching, C. K., Tang, B. S. F., Lau, C. C. Y., Li, I. W. S., Liu, S. H., Chan, K. H., Lin, C. K., & Yuen, K. Y. (2011). Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clinical Infectious Diseases, 52(4), 447–456.
Lai, S. T. (2005). Treatment of severe acute respiratory syndrome. European Journal of Clinical Microbiology & Infectious Diseases, 24(9), 583–591.
Cheng, Y., Wong, R., Soo, Y. O. Y., Wong, W. S., Lee, C. K., Ng, M. H. L., Chan, P., Wong, K. C., Leung, C. B., & Cheng, G. (2005). Use of convalescent plasma therapy in SARS patients in Hong Kong. European Journal of Clinical Microbiology & Infectious Diseases, 24(1), 44–46.
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-continues-facilitate-development-treatments. [Accessed: 25-Mar-2020]., C.C.-U.F.C.t.F.D.o.T.F.O.A.
Beigel, J. H., Voell, J., Kumar, P., Raviprakash, K., Wu, H., Jiao, J. A., Sullivan, E., Luke, T., & Davey Jr., R. T. (2018). Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: A phase 1 randomised, double-blind, single-dose-escalation study. The Lancet Infectious Diseases, 18(4), 410–418.
Shanmugaraj, B., Siriwattananon, K., Wangkanont, K., & Phoolcharoen, W. (2020). Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19). Asian Pacific Journal of Allergy and Immunology, 38(1), 10–18.
Kuba, K., Imai, Y., Rao, S., Gao, H., Guo, F., Guan, B., Huan, Y., Yang, P., Zhang, Y., Deng, W., Bao, L., Zhang, B., Liu, G., Wang, Z., Chappell, M., Liu, Y., Zheng, D., Leibbrandt, A., Wada, T., Slutsky, A. S., Liu, D., Qin, C., Jiang, C., & Penninger, J. M. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine, 11(8), 875–879.
Saghazadeh, A., & Rezaei, N. (2020). Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti-antibodies, immunoglobulins, and corticosteroids. International Immunopharmacology, 84, 106560.
Zumla, A., Chan, J. F. W., Azhar, E. I., Hui, D. S. C., & Yuen, K.-Y. (2016). Coronaviruses — Drug discovery and therapeutic options. Nature Reviews Drug Discovery, 15, 327–347 Nature Publishing Group, (February 2003).
Dillman, R. O. (2011). Cancer immunotherapy. Cancer Biotherapy & Radiopharmaceuticals, 26(1), 1–64.
Schuster, M., Nechansky, A., & Kircheis, R. (2006). Cancer immunotherapy. Biotechnology Journal, 1(2), 138–147.
Mok, M. Y., & Shoenfeld, Y. (2016). Recent advances and current state of immunotherapy in systemic lupus erythematosus. Expert Opinion on Biological Therapy, 16(7), 927–939.
Karim, M. Y., Pisoni, C. N., & Khamashta, M. A. (2009). Update on immunotherapy for systemic lupus erythematosus—what's hot and what's not! Rheumatology, 48(4), 332–341.
Fong, K. Y. (2002). Immunotherapy in autoimmune diseases. Annals of the Academy of Medicine, Singapore, 31(6), 702–706.
Naran, K., Nundalall, T., Chetty, S., & Barth, S. (2018). Principles of immunotherapy: Implications for treatment strategies in Cancer and infectious diseases. Frontiers in Microbiology, 9, 3158–3158.
Qin, C., et al. (2020). Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases, https://doi.org/10.1093/cid/ciaa248.
SGV, R., & Santos, W. C. (2020). Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana de Salud Pública, 44(40), e40.
de Mendonca, L., et al. (2017). Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Research & Therapy, 8(1), 220.
Chen, J., Hu, C., Chen, L., Tang, L., Zhu, Y., Xu, X., Chen, L., Gao, H., Lu, X., Yu, L., Dai, X., Xiang, C., & Li, L. (2020), Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering.
Geiger, S., Hirsch, D., & Hermann, F. G. (2017). Cell therapy for lung disease. European Respiratory Review, 26(144), 170044.
Lukomska, B., Stanaszek, L., Zuba-Surma, E., Legosz, P., Sarzynska, S., & Drela, K. (2019). Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells International, 2019, 1–10.
Squillaro, T., Peluso, G., & Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: An update. Cell Transplantation, 25(5), 829–848.
Uccelli, A., & de Rosbo, N. K. (2015). The immunomodulatory function of mesenchymal stem cells: Mode of action and pathways. Annals of the New York Academy of Sciences, 1351(1), 114–126.
Ben-Mordechai, T., Palevski, D., Glucksam-Galnoy, Y., Elron-Gross, I., Margalit, R., & Leor, J. (2015). Targeting macrophage subsets for infarct repair. Journal of Cardiovascular Pharmacology and Therapeutics, 20(1), 36–51.
Cardenes, N., Aranda-Valderrama, P., Carney, J. P., Sellares Torres, J., Alvarez, D., Kocydirim, E., Wolfram Smith, J. A., Ting, A. E., Lagazzi, L., Yu, Z., Mason, S., Santos, E., Lopresti, B. J., & Rojas, M. (2019). Cell therapy for ARDS: Efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respiratory Research, 6(1), e000308.
Zheng, G., Huang, L., Tong, H., Shu, Q., Hu, Y., Ge, M., Deng, K., Zhang, L., Zou, B., Cheng, B., & Xu, J. (2014). Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study. Respiratory Research, 15(1), 39.
Gebler, A., Zabel, O., & Seliger, B. (2012). The immunomodulatory capacity of mesenchymal stem cells. Trends in Molecular Medicine, 18(2), 128–134.
Soleymaninejadian, E., Pramanik, K., & Samadian, E. (2012). Immunomodulatory properties of mesenchymal stem cells: Cytokines and factors. American Journal of Reproductive Immunology, 67(1), 1–8.
Galipeau, J., & Sensébé, L. (2018). Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell, 22(6), 824–833.
Uccelli, A., Pistoia, V., & Moretta, L. (2007). Mesenchymal stem cells: A new strategy for immunosuppression? Trends in Immunology, 28(5), 219–226.
Ribeiro, A., Laranjeira, P., Mendes, S., Velada, I., Leite, C., Andrade, P., Santos, F., Henriques, A., Grãos, M., Cardoso, C. M. P., Martinho, A., Pais, M. L., da Silva, C., Cabral, J., Trindade, H., & Paiva, A. (2013). Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Research & Therapy, 4(5), 125.
Tomchuck, S. L., Zwezdaryk, K. J., Coffelt, S. B., Waterman, R. S., Danka, E. S., & Scandurro, A. B. (2008). Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells, 26(1), 99–107.
Pevsner-Fischer, M., Morad, V., Cohen-Sfady, M., Rousso-Noori, L., Zanin-Zhorov, A., Cohen, S., Cohen, I. R., & Zipori, D. (2007). Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood, 109(4), 1422–1432.
Leng, Z., Zhu, R., Hou, W., Feng, Y., Yang, Y., Han, Q., et al. (2020). Transplantation of ACE2− Mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease, 11(2), 216–228.
Fathi, N. & Rezaei, N. (2020). Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biology International, 1–6.
Bari, E., Ferrarotti, I., Saracino, L., Perteghella, S., Torre, M. L., & Corsico, A. G. (2020). Mesenchymal stromal cell secretome for severe COVID-19 infections: Premises for the therapeutic use. Cells, 9(4), 924.
Lee, J. W., Fang, X., Krasnodembskaya, A., Howard, J. P., & Matthay, M. A. (2011). Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells, 29(6), 913–919.
Matthay, M. A., Thompson, B. T., Read, E. J., McKenna Jr., D. H., Liu, K. D., Calfee, C. S., & Lee, J. W. (2010). Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest, 138(4), 965–972.
Shetty, A. K. (2020). Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging and Disease, 11(2), 462–464.
Aghaeepour, N., et al. (2017). An immune clock of human pregnancy. Science Immunology, 2(15), eaan2946.
Smyth, M. J., Hayakawa, Y., Takeda, K., & Yagita, H. (2002). New aspects of natural-killer-cell surveillance and therapy of cancer. Nature Reviews Cancer, 2(11), 850–861.
Saghazadeh, A., & Rezaei, N. (2017). Implications of toll-like receptors in Ebola infection. Expert Opinion on Therapeutic Targets, 21(4), 415–425.
Fehniger, T. A., & Cooper, M. A. (2016). Harnessing NK cell memory for cancer immunotherapy. Trends in Immunology, 37(12), 877–888.
Vivier, E., Raulet, D. H., Moretta, A., Caligiuri, M. A., Zitvogel, L., Lanier, L. L., Yokoyama, W. M., & Ugolini, S. (2011). Innate or adaptive immunity? The example of natural killer cells. Science, 331(6013), 44–49.
O’Sullivan, T. E., Sun, J. C., & Lanier, L. L. (2015). Natural killer cell memory. Immunity, 43(4), 634–645.
Van Niel, G., d’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19(4), 213–228.
Biancone, L., Bruno, S., Deregibus, M. C., Tetta, C., & Camussi, G. (2012). Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrology Dialysis Transplantation, 27(8), 3037–3042.
Akbari, A., Jabbari, N., Sharifi, R., Ahmadi, M., Vahhabi, A., Seyedzadeh, S. J., Nawaz, M., Szafert, S., Mahmoodi, M., Jabbari, E., Asghari, R., & Rezaie, J. (2020). Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sciences, 249, 117447.
Chen, T. S., Lai, R. C., Lee, M. M., Choo, A. B. H., Lee, C. N., & Lim, S. K. (2010). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research, 38(1), 215–224.
Lai, R. C., Tan, S. S., Teh, B. J., Sze, S. K., Arslan, F., de Kleijn, D. P., Choo, A., & Lim, S. K. (2012). Proteolytic potential of the MSC exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. International journal of proteomics, 2012, 1–14.
Burrello, J., et al. (2016). Stem cell-derived extracellular vesicles and immune-modulation. Frontiers in Cell and Developmental Biology, 4, 83.
Khatri, M., Richardson, L. A., & Meulia, T. (2018). Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Research & Therapy, 9(1), 1–13.
Cruz, F. F., Borg, Z. D., Goodwin, M., Sokocevic, D., Wagner, D. E., Coffey, A., Antunes, M., Robinson, K. L., Mitsialis, S. A., Kourembanas, S., Thane, K., Hoffman, A. M., McKenna, D. H., Rocco, P. R. M., & Weiss, D. J. (2015). Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine, 4(11), 1302–1316.
Lanyu, Z., & Feilong, H. (2019). Emerging role of extracellular vesicles in lung injury and inflammation. Biomedicine & Pharmacotherapy, 113, 108748.
Kuate, S., Cinatl, J., Doerr, H. W., & Überla, K. (2007). Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology, 362(1), 26–37.
Wang, Y.-D., Li, Y., Xu, G. B., Dong, X. Y., Yang, X. A., Feng, Z. R., Tian, C., & Chen, W. F. (2004). Detection of antibodies against SARS-CoV in serum from SARS-infected donors with ELISA and Western blot. Clinical Immunology, 113(2), 145–150.
Wilkinson, D. C., Alva-Ornelas, J. A., Sucre, J. M. S., Vijayaraj, P., Durra, A., Richardson, W., Jonas, S. J., Paul, M. K., Karumbayaram, S., Dunn, B., & Gomperts, B. N. (2017). Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Translational Medicine, 6(2), 622–633.
Sundarakrishnan, A., Chen, Y., Black, L. D., Aldridge, B. B., & Kaplan, D. L. (2018). Engineered cell and tissue models of pulmonary fibrosis. Advanced Drug Delivery Reviews, 129, 78–94.
Ebert, A. D., & Svendsen, C. N. (2010). Human stem cells and drug screening: Opportunities and challenges. Nature Reviews Drug Discovery, 9(5), 367–372.
Wilkinson, D. C., Mellody, M., Meneses, L. K., Hope, A. C., Dunn, B., & Gomperts, B. N. (2018). Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Current Protocols in Stem Cell Biology, 46(1), e56.
Porotto, M., et al. (2019). Authentic modeling of human respiratory virus infection in human pluripotent stem cell-derived lung Organoids. MBio, 10(3), e00723–e00719.
Kilianski, A., & Baker, S. C. (2014). Cell-based antiviral screening against coronaviruses: Developing virus-specific and broad-spectrum inhibitors. Antiviral Research, 101, 105–112.
Boda, B., Benaoudia, S., Huang, S., Bonfante, R., Wiszniewski, L., Tseligka, E. D., Tapparel, C., & Constant, S. (2018). Antiviral drug screening by assessing epithelial functions and innate immune responses in human 3D airway epithelium model. Antiviral Research, 156, 72–79.
Ge, F., Luo, Y., Liew, P. X., & Hung, E. (2007). Derivation of a novel SARS–coronavirus replicon cell line and its application for anti-SARS drug screening. Virology, 360(1), 150–158.
Rezaei, N. (2020). COVID-19 affects healthy pediatricians more than pediatric patients. Infection Control & Hospital Epidemiology, 1.
Moradian, N., et al. (2020). The urgent need for integrated science to fight COVID-19 pandemic and beyond. Journal of Translational Medicine, 18(1), 1–7.
Mohamed, K., Rodríguez-Román, E., Rahmani, F., Zhang, H., Ivanovska, M., Makka, S. A., et al. (2020). Borderless collaboration is needed for COVID-19—A disease that knows no borders. Infection Control & Hospital Epidemiology, 1–2.
Momtazmanesh, S., et al. (2020). All together to fight novel coronavirus disease (COVID-19). The American Journal of Tropical Medicine and Hygiene, tpmd200281.
Saleki, K., et al. (2020). The involvement of the central nervous system in patients with COVID-19. Reviews in the Neurosciences, 1(ahead-of-print).
Jahanshahlu, L. & Rezaei, N. (2020). Central nervous system involvement in COVID-19. Archives of Medical Research.
Nasab, M. G., Saghazadeh, A., & Rezaei, N. (2020). SARS-CoV-2–A tough opponent for the immune system. Archives of Medical Research.
Golchin, A., Seyedjafari, E., & Ardeshirylajimi, A. (2020). Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Reviews and Reports, 16(3), 427–433.
http://www.chictr.org.cn.
clinicaltrials.gov.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arefeh Basiri and Zahra Pazhouhnia are co-first authors.
Rights and permissions
About this article
Cite this article
Basiri, A., Pazhouhnia, Z., Beheshtizadeh, N. et al. Regenerative Medicine in COVID-19 Treatment: Real Opportunities and Range of Promises. Stem Cell Rev and Rep 17, 163–175 (2021). https://doi.org/10.1007/s12015-020-09994-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09994-5