Abstract
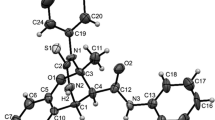
Cancer is often associated with chronic inflammation. In order to develop potential anticancer and anti-inflammatory agents a series of 26 diarylidenecyclopentanones (DACPs) Ia–Iv, II, III, and IV were synthesized. Five of the synthesized DACPs are novel (Ih, Ij, Ik, Is, and Iv), derivative Iv was characterized using single-crystal X-ray diffraction study. All the synthesized derivatives were tested for their anti-inflammatory as well as cytotoxicity properties. Compound Is is found to have the highest anti-inflammatory activity (93.67%) by inhibiting PGE2 (prostaglandin E2) production. Three of the DACPs (Io, It, and Iu) were observed to have high cytotoxicity with IC50 value of 8.73 ± 0.06 µM (Io), 12.55 ± 0.31 µM (It), and 11.47 ± 0.15 µM (Iu) against HeLa cells. Further staining and cell cycle analysis was done using these three DACPs to understand their mechanism of action. The G0/G1 phase was observed to be the longest one through which the cells undergo apoptosis.










Similar content being viewed by others
Abbreviations
- AO:
-
Acridine orange
- COX:
-
cyclooxygenase
- DACP:
-
Diarylidenecyclopenatnone
- DMEM:
-
Dulbecco's Modified Eagle Medium
- ETBR:
-
Ethidium bromide
- mPGES-1:
-
membrane-associated PGE synthase-1
- MTT:
-
3-(4,5- dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
- NCCS:
-
National Centre for Cell Science
- NCI:
-
National Cancer Institute
- PBS:
-
phosphate buffered saline
- PGE2:
-
Prostaglandin E2
- PI:
-
Propidium Iodide
References
Axelrad JE, Lichtiger S, Yajnik V (2016) Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 22:4794
Chen H, Xu X, Wang G, Zhang B, Wang G, Xin G, Liu J, Jiang Q, Zhang H, Zhang C (2017) CDK4 protein is degraded by anaphase-promoting complex/cyclosome in mitosis and reaccumulates in early G1 phase to initiate a new cell cycle in HeLa cells. J Biol Chem 292:10131–10141
Das M, Manna K (2016) Chalcone scaffold in anticancer armamentarium: a molecular insight. J Toxicol 2016:1–14. https://doi.org/10.1155/2016/7651047
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341
Du ZY, Liu RR, Shao WY, Mao XP, Ma L, Gu LQ, Huang ZS, Chan AS (2006) α-Glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur J Med Chem 41:213–218
Eiró N, Vizoso FJ (2012) Inflammation and cancer. World J Gastrointest Surg 4:62
Ghosh N, Chaki R, Mandal V, Mandal SC (2010) COX-2 as a target for cancer chemotherapy. Pharmacol Rep 62(2):233–244
Jia H, Pan G, Wang Y, Wen S, Guo Q, Hu W, Yu P, Sun H, Teng Y (2014) Design, synthesis and primary biological evaluation of the novel antitumor agent indoline-3-one and its derivatives. In: Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012). pp 875–882
Joshi BP, Mohanakrishnan D, Mittal G, Kar S, Pola JK, Golakoti NR, Nanubolu JB, Babu R, and Sahal D (2018) Synthesis, mechanistic and synergy studies of diarylidenecyclohexanone derivatives as new antiplasmodial pharmacophores. Med Chem Res 2312–2324. https://doi.org/10.1007/s00044-018-2237-2
Kar S, Mishra RK, Pathak A, Dikshit A, Golakoti NR (2018) In silico modeling and synthesis of phenyl and thienyl analogs of chalcones for potential leads as anti-bacterial agents. J Mol Struct 1156:433–440
Kar S, Ramamoorthy G, Sinha S, Ramanan M, Pola JK, Golakoti NR, Nanubolu JB, Sahoo SK, Dandamudi RB, Doble M (2019) Synthesis of diarylidenecyclohexanone derivatives as potential anti-inflammatory leads against COX-2/mPGES1 and 5-LOX. New J Chem 9012–9020. https://doi.org/10.1039/C9NJ00726A
Kauerova T, Kos J, Gonec T, Jampilek J, Kollar P (2016) Antiproliferative and pro-apoptotic effect of novel nitro-substituted hydroxynaphthanilides on human cancer cell lines. Int J Mol Sci 17:1219
Khazaei S, Esa NM, Ramachandran V, Hamid RA, Pandurangan AK, Etemad A, Ismail P (2017) In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front Pharm 8:5
Lee KH, Aziz FH, Syahida A, Abas F, Shaari K, Israf DA, Lajis NH (2009) Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur J Med Chem 44:3195–3200
Liang G, Li X, Chen L, Yang S, Wu X, Studer E, Gurley E, Hylemon PB, Ye F, Li Y, Zhou H (2008) Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. BMC Lett 18:1525–1529. https://doi.org/10.1016/j.bmcl.2007.12.068
Liang G, Shao L, Wang Y, Zhao C, Chu Y, Xiao J, Zhao Y, Li X, Yang S (2009a) Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem 17:2623–2631. https://doi.org/10.1016/j.bmc.2008.10.044
Liang G, Yang S, Jiang L, Zhao Y, Shao L, Xiao J, Ye F, Li Y, Li X (2008) Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull 56:162–167
Liang G, Zhou H, Wang Y, Gurley EC, Feng B, Chen L, Xiao J, Yang S, Li X (2009b) Inhibition of LPS-induced production of inflammatory factors in the macrophages by mono-carbonyl analogues of curcumin. J Cell Mol Med 13:3370–3379. https://doi.org/10.1111/j.1582-4934.2009.00711.x
Lima RS, Perez CN, da Silva CC, Santana MJ, Junior LH, Barreto S, de Moraes MO, Martins FT (2016) Structure and cytotoxic activity of terpenoid-likechalcones. Arab J Chem 12(8):3890–3901
Liu GY, Jia CC, Han PR, Yang J (2018) 3,5-Bis(2-fluorobenzylidene)-4-piperidone induce reactive oxygen species-mediated apoptosis in A549 cells. Med Chem Res 128–136. https://doi.org/10.1007/s00044-017-2056-x
Liu Z, Tang L, Zou P, Zhang Y, Wang Z, Fang Q, Jiang L, Chen G, Xu Z, Zhang H, Liang G (2014) Synthesis and biological evaluation of allylated and prenylated mono-carbonyl analogs of curcumin as anti-inflammatory agents. Eur J Med Chem 74:671–682. https://doi.org/10.1016/j.ejmech.2013.10.061
Lorusso G, Rüegg C (2008) The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 130:1091–1103
Luo H, Wang F, Bai Y, Chen T, Zheng W (2012) Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf B Biointerfaces 94:304–308
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, Streek JV, Wood PA (2008) Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J Appl Crystallogr 41:466–470
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 436–444
Nakanishi M, Gokhale V, Meuillet EJ, Rosenberg DW (2010) mPGES-1 as a target for cancer suppression: a comprehensive invited review “Phospholipase A2 and lipid mediators”. Biochimie 660–664.
National Cancer Institute (NCI) (2020) https://www.cancer.gov/about-cancer/treatment/types Accessed 18 Mar 2020
Pan Z, Chen C, Zhou Y, Xu F, Xu Y (2016) Synthesis and cytotoxic evaluation of monocarbonyl analogs of curcumin as potential anti-tumor agents. Drug Dev Res 77:43–49
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G (2016) Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging 8:603
Puratchikody A, Umamaheswari A, Irfan N, Sinha S, Manju SL, Ramanan M, Ramamoorthy G, Doble M (2019) A novel class of tyrosine derivatives as dual 5-LOX and COX-2/mPGES1 inhibitors with PGE2 mediated anticancer properties. N J Chem 43:834–846
Putri TN, Bachtiar A, Hayun H (2018) Synthesis, antioxidant, and anti-inflammatory activity of morpholine Mannich base of AMACs ((2 E, 6 E) -2- ({ 4-hydroxy-3- cyclohexan-1-one) and its analogs. 8:19–25. https://doi.org/10.7324/JAPS.2018.8503
Qian Y, Zhong P, Liang D, Xu Z, Skibba M, Zeng C, Li X, Wei T, Wu L, Liang G (2015) A newly designed curcumin analog Y20 mitigates cardiac injury via anti- inflammatory and anti-oxidant actions in obese rats. 1–16. https://doi.org/10.1371/journal.pone.0120215
Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol 5:12
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr Sect A Found Crystallogr 64:112–122
Shin SY, Yong Y, Kim CG, Lee YH, Lim Y (2010) Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett 287:231–239
Silva Lopes M, Filizzola de Andrade Sena C, Leonardo Silva B, Maria de Souza C, Pereira Ramos J, Dantas Cassali G, Maria de Souza-Fagundes E, Jose Alves R, Cristina de Oliveira M, Barbosa, de Oliveira R (2015) Synthesis of nitroaromatic compounds as potential anticancer agents. Anti-Cancer Agents Med Chem 15:206–216
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904
Tandon VK, Maurya HK, Tripathi A, ShivaKeshava GB, Shukla PK, Srivastava P, Panda D (2009) 2, 3-Disubstituted-1, 4-naphthoquinones, 12H-benzo [b] phenothiazine-6, 11-diones and related compounds: synthesis and biological evaluation as potential antiproliferative and antifungal agents. Eur J Med Chem 44:1086–1092
Wang Y, Yu C, Pan Y, Yang X, Huang Y, Feng Z, Li X, Yang S, Liang G (2012) A novel synthetic mono-carbonyl analogue of curcumin, A13, exhibits anti-inflammatory effects in vivo by inhibition of inflammatory mediators Inflammation 35:594–604. https://doi.org/10.1007/s10753-011-9350-4
Wang Y, Yu H, Zhang J, Gao J, Ge X, Lou G (2015) Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 15:682
Wiji Prasetyaningrum P, Bahtiar A, Hayun H (2018) Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci Pharm 86:25
World Health Organisation (WHO) (2018) https://who.int/news-room/fact-sheets/detail/cancer. Accessed 18 Mar 2020
Wu J, Zhang Y, Cai Y, Wang J, Weng B, Tang Q, Chen X, Pan Z, Liang G, Yang S (2013) Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg Med Chem 21:3058–3065. https://doi.org/10.1016/j.bmc.2013.03.057
Zhao C, Yang J, Wang Y, Liang D, Yang X, Li X, Wu J, Wu X, Yang S, Li X, Liang G (2010) Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg Med Chem 18:2388–2393. https://doi.org/10.1016/j.bmc.2010.03.001
Zhao C, Zhang Y, Zou P, Wang J, He W, Shi D, Li H, Liang G, Yang S (2015) Synthesis and biological evaluation of a novel class of curcumin analogs as anti-inflammatory agents for prevention and treatment of sepsis in mouse model. Drug Des Devel Ther 9:1663–1678
Acknowledgements
Authors dedicate the work to Founder Chancellor Bhagawan Sri Sathya Sai Baba. The authors thank CRIF, SSSIHL for providing the characterization facilities. We also thank Sri Swayamsiddha Kar and Dr. Sai Manohar Chelli for their valuable inputs. Nitesh Tamang thanks MOTA for the NFST fellowship (201819-NFST-WES-02502) and Mayank Joshi thanks DST for Inspire fellowship (IF160793).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nitesh Tamang, Gayathri Ramamoorthy
Supplementary information
Rights and permissions
About this article
Cite this article
Tamang, N., Ramamoorthy, G., Joshi, M. et al. Diarylidenecyclopentanone derivatives as potent anti-inflammatory and anticancer agents. Med Chem Res 29, 1579–1589 (2020). https://doi.org/10.1007/s00044-020-02578-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02578-5