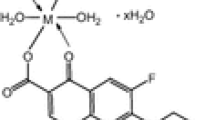
Abstract
Purpose
The aim of this work is to study the metal–drug–amino acid ternary complex formation kinetically, thermodynamically and computationally and explore the possibility of developing some potent new drugs for biological applications.
Methods
Metal(II)–ciprofloxacin–glycine ternary complexes (metal = MnII, CuII, ZnII) were synthesized. The slow kinetics and thermodynamics of imine formation were investigated spectrophotometrically. The geometry optimizations of ternary complexes were studied by the density functional theory (DFT) using TURBOMOL 6.5 software. The in vitro antibacterial activities of the ternary complexes against gram-positive and gram-negative bacteria were evaluated by disc diffusion method.
Results
It turns out that Cu(II) is the most and Mn(II) is the least efficient in terms of thermodynamics control, while Zn(II) is the most active and Cu(II) is the least active in terms of kinetic control of the ternary imine complex formation, a consequence of kinetic–coordination–template process. The biological screening showed that the ternary metal complexes are as active as the parent drug (cf). However, the uncharged ternary complex may be better career of the drug to the active site due to the presence of metal ion, entailing further detailed investigation.
Conclusion
Kinetics, thermodynamic and in vitro antibacterial activities of metal complexes were investigated. Metal complexes show similar activity towards bacteria as free drug, but they have several advantages over the neat drug (cf), such that increase in lipophilicity of the ternary complex enhances the passage into the cell wall of the microorganism more efficiently than the free drug and blocks the growth of organism; increases the time of post-bacterial activity; and meets the deficiency of metals Cu, Zn, Mn and glycine (gly) which are essential for different metabolisms in biological domain.














Similar content being viewed by others
References
Albert AD. The physico-chemical basic of therapy: selective toxics. 6th ed. London: Chapman and Hall; 2005.
Reynolds JEF, editor. The extra pharmacopoia. 30th ed. London: The Pharmaceutical Press; 1993.
Nehu HC. Am J Med. 1987;82:395–404.
Liu B, Nie X, Liu W, Snoeijs P, Guan C, Tsui MTK. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol Environ Saf. 2011;74:1027–35. https://doi.org/10.1016/j.ecoenv.2011.01.022.
Turel I, Golobic A, Klavzar A, Pihlar B, Buglyo P, Tolis E, et al. Interactions of oxovanadium(IV) and the quinolone family member—ciprofloxacin. J Inorg Biochem. 2003;95:199–207. https://doi.org/10.1016/S0162-0134(03)00123-5.
Psomas G. Mononuclear metal complexes with ciprofloxacin: synthesis, characterization and DNA-binding properties. J Inorg Biochem. 2008;102(9):1798–811. https://doi.org/10.1016/j.jinorgbio.2008.05.012.
Turel I. The interactions of metal ions with quinolone antibacterial agents. Coord Chem Rev. 2002;232:27–47. https://doi.org/10.1016/S0010-8545(02)00027-9.
Muthumariappan S. Synthesis and characterization of ciprofloxacin–zinc(II) complex and assay studies in pharmaceutical drugs. J Pharm Res. 2013;6(4):437–41. https://doi.org/10.1016/j.jopr.2013.04.009.
Rizzi GP, Amba E, Heineman WR. Quantification of chemically reducing species in the phosphate ion catalyzed degradation of reducing sugars. J Agric and Food Chem. 2010;58(17):9739–43. https://doi.org/10.1021/if1024334
Dash AC, Nanda RK. Reactions of coordinated ligands. I. Kinetics and mechanism hydrolysis of N-salicylideneaniline in the presence of metal ions. J Am Chem Soc. 1969;91:6944–7. https://doi.org/10.1021/ja01053a010.
Dash AC, Dash B, Praharaj SJ. Hydrolysis of imines: kinetics and mechanism of spontaneous, acid, base and metal-ion induced hydrolysis of N-salicylidene-2-amino thiazole. CS Dalton. 1981;2063–69.
Dash AC, Patra M, Dash B, Mahapatra PK. Hydrolysis of imines. Part 2. Kinetics and mechanism of hydrolysis of N-salicylidene-2-aminopyridine in the presence and absence of copper(II) ion. A study of the catalytic effect of some mixed-ligand complexes of copper(II). J Chem Soc Dalton Trans. 1983;1503–1509.
Dash AC, Dash B, Panda D. Hydrolysis of imines. Micellar effects upon the spontaneous acid, base, and copper(II) ion induced hydrolysis of N-salicylidene-2-aminothiazole and N-salicylidene-2-aminopyridine. J Org Chem. 1985;50:2905–10 0022-3263/85/1950-2905$01.50/0.
Dash AC, Dash B, Panda D. Micellar effect on the hydrolysis of imines: kinetics and mechanism of alkaline hydrolysis of N-salicylidene-2-amino thiazole and N-salicylidene-2-amino pyridine in the presence of cetyl trimethyl ammonium bromide. J Surf Sci Tech. 1985;1:59–62
Dash AC, Panda D, Dash B. Kinetics & equilibrium studies on reversible formation of N-salicylidene 2-aminomethylbenzimidazole & its copper(II) complex. Indian J Chem. 1986;25A:141–6. http://nopr.niscair.res.in/handle/123456789/48099.
Dash AC, Acharya AN, Sahoo RK. Binary and ternary complexes of nickel(II) 2-aminomethylbenzimidazole and salicylaldehyde: kinetic and equilibrium studies. J Chem Soc Dalton Trans. 1994;3727–3731. https://doi.org/10.1039/DT9940003727.
Das NN, Dash AC. Synthesis, characterization and electrochemistry of a binuclear copper(II) complex of Schiff base derived from 2-aminomethyl-benzimidazole and salicylaldehyde. Polyhedron. 1995;14(9):1221–7. https://doi.org/10.1016/0277-5387(94)00374-N.
Vogel AI. A text book of quantitative inorganic analysis. 4th ed. London: ELBS and Longan; 1978. p. 392.
Vogel AI. A text book on practical organic chemistry including qualitative organic analysis. 4th ed. London: Longman Group Ltd.; 1978. p. 264–9.
Irving H, Williams R. Order of stability of metal complexes. Nature. 1948;162:746. https://doi.org/10.1038/162746a0.
Pelczar MJ, Chan ECS, Kreig NR. Microbiology. 5th ed. New York: Black Well Science; 1948.
Acknowledgements
The authors are thankful to Prof. A. C. Dash (Retd.Professor, Utkal University) for his constant guidance for completion of this paper. One of the authors, J Panda, is thankful to the Head of the Department of Chemistry, Utkal University, Bhubaneswar, for providing research facilities and also thankful to UGC and Govt. of Odisha for sanctioning teacher fellowship. The authors express their gratitude to Dr. Sharanappa Nembenna, Scientist-F, NISER, Odisha, for his whole-hearted cooperation for mass and NMR spectral analysis and Dr. Himansu Sekhar Biswala, Associate Prof. Chemistry, NISER, Odisha, for computational analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
The research was done without use of any animal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panda, J., Das, S., Patnaik, A.K. et al. Interaction of Metal(II) Ion–Ciprofloxacin–Glycine in Solution. J Pharm Innov 16, 454–468 (2021). https://doi.org/10.1007/s12247-020-09451-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09451-3