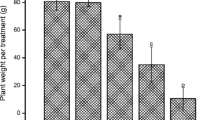
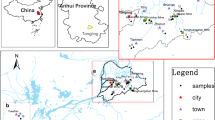
Abstract
Antimony is a toxic element whose concentration in soil and water has been rising due to anthropogenic activities. This study focuses on its accumulation in leaves of Dittrichia viscosa growing in soils of an abandoned Sb mine, and the effect on oxidant/antioxidant systems and photosynthetic efficiency. The results showed leaves to have a high Sb accumulation capacity. The amount of total chlorophyll decreased depending on Sb concentration and of carotenoids increased slightly, with a consequent increase in carotenoid/chlorophyll ratio. Photosynthetic efficiency was unaffected. The amount of O .−2 rose, although there was no increase in cell membrane damage, with lipid peroxidation levels being similar to normal. This response may be due to considerable increases that were observed in total phenolics, PPO activity, and enzymatic antioxidant system. SOD, POX, and DHAR activities increased in response to increased Sb amounts in leaves. The ascorbate/glutathione cycle was also affected, with strong increases observed in all of its components, and consequent increases in total contents of the ascorbate and glutathione pools. However, the ratio between reduced and oxidized forms declined, reflecting an imbalance between the two, especially that between GSH and GSSG. Efficient detoxification of Sb may take place either through increases in phenolics, carotenoids, and components of the glutathione–ascorbate cycle or through the enzymatic antioxidant system. Since Dittrichia viscosa accumulates large amounts of Sb without suffering oxidative damage, it could be used for phytoremediation.



Similar content being viewed by others
References
Agati, G., Azzarello, E., Pollastri, S., & Tattini, M. (2012). Flavonoids as antioxidants in plants: Location and functional significance. Plant Science, 196, 67–76.
Álvarez-Ayuso, E., Otones, V., Murciego, A., García-Sánchez, A., & Santa, Regina I. (2013). Mobility and phytoavailability of antimony in an area impacted by a former stibnite mine exploitation. Science of the Total Environment, 449, 260–268.
Anjum, N. A., Gill, S. S., Gill, R., Hasanuzzaman, M., Duarte, A. C., Pereira, E., et al. (2014). Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma, 251, 1265–1283.
Anjum, N. A., Umar, S., Iqbal, M., & Khan, N. A. (2011). Cadmium causes oxidative stress in moongbean [Vigna radiata (L.) Wilcaek] by affecting antioxidant enzyme systems and ascorbate-glutathione cycle metabolism. Russian Journal of Plant Physiology, 58, 92–99.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biololgy, 55, 373–399.
Baroni, F., Boscagli, A., Protano, G., & Riccobono, F. (2000). Antimony accumulation in Achillea ageratum, Plantago lanceolata and Silene vulgaris growing in an old Sb-mining area. Environmental Pollution, 109, 347–352.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Benhamdi, A., Bentellis, A., Rached, O., Du Laing, G., & Mechakra, A. (2014). Effects of antimony and arsenic on antioxidant enzyme activities two steppic plant species in an old antimony mining area. Biological Trace Elements Research, 158, 96–104.
Blake, G. R., & Hartge, K. H. (1986) Bulk density. In: Klute, A., (Ed.), Methods of soil analysis, part 1. Physical and mineralogical methods, 2nd edn, Agronomy Monograph 9, American Society of Agronomy-Soil Science Society of America, Madison (pp. 363–382).
Bowen, H. J. M. (1979). Environmental chemistry of the elements. London: Academic Press.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Buck, R. P., Rondinini, S., Covington, A. K., Baucke, F. G. K., Brett, C. M. A., Camões, M. F., et al. (2002). Measurement of pH. Definition, standards, and procedures (IUPAC Recommendations 2002). Pure Applied Chemistry, 74(11), 2169–2200.
Cai, F., Ren, J., Tan, S., & Wang, S. (2016). Uptake, translocation and transformation of antimony in rice (Oryza sativa L.) seedling. Environmental Pollution, 209, 169–176.
Chai, L. Y., Mubarak, H., Yang, Z. H., Yong, W., Tang, C. J., & Mirza, N. (2016). Growth, photosynthesis and defense mechanism of antimnoy (Sb)-contaminated Boehmeria nivea (L). Environmental Science Pollution Research, 23(8), 7470–7481.
Chai, L. Y., Wang, Y., Yang, Z. H., Mubarak, H., & Mirza, N. (2017). Physiological characteristics of Ficus tikoua under antimony stress. Transations of Nonferrous Metals Society of China, 27, 939–945.
Cidu, R., Biddau, R., Dore, E., Vacca, A., & Marini, L. (2014). Antimony in the soil–water–plant system at the Su Suergiu abandoned mine (Sardinia, Italy): Strategies to mitigate contamination. Science of the Total Environment, 497–498, 319–331.
Clemente, R. (2013). Antimony. In B. J. Alloway (Ed.), Heavy metals in soils, trace metals and metalloids in soils and their bioavailability (3rd ed., pp. 494–506). Dordrecht: Springer.
Corrales, I., Barceló, J., Bech, J., & Poschenrieder, C. (2014). Antimony accumulation and toxicity tolerance mechanisms in Trifolium species. Journal of Geochemical Exploration, 147, 167–172.
Cos, P., Rajan, P., Vedernikiva, I., Calomme, M., Pieters, L., Vlietinck, A. J., et al. (2002). In vitro antioxidant profile of phenolic acid derivatives. Free Radical Research, 36, 711–716.
De Gara, L., De Pinto, M. C., Moliterni, V. M. C., & D’Egidio, M. G. (2003). Redox regulation and storage processes during maturation in kernels of Triticum durum. Journal of Experimental Botany, 54, 249–258.
De Pinto, M. C., Francis, D., & De Gara, L. (1999). The redox state of ascorbate-dehydroascorbate pair as a specific sensor of cell división in tobacco TBY-2 cells. Protoplasma, 209, 90–97.
Elliot, A. J., Scheiber, S. A., Thomas, C., & Pardini, R. S. (1992). Inhibition of glutathione reductase by flavonoids. A structure-activity study. Biochemical Pharmacology, 44(8), 1603–1608.
Feigl, G., Lehotai, N., Molnár, A., Ördög, A., Rodríguez-Ruíz, M., Palma, J. M., et al. (2015). Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Annals of Botany, 116, 613–625.
Feng, R., Wei, C., Tu, S., Ding, Y., Wang, R., & Guo, J. (2013). The uptake and detoxification of antimony by plants: A review. Environmental and Experimental Botany, 96, 28–34.
Feng, R., Wei, C., Tu, S., Wu, F., & Yang, L. (2009). Antimony accumulation and antioxidative responses in four fern plants. Plant and Soil, 317, 93–101.
Filella, M., Belzile, N., & Chen, Y. W. (2002). Antimony in the environment: A review focused on natural waters: I. Ocurrence. Earth-Science Reviews, 57(1–2), 125–176.
Filella, M., Williams, P. A., & Belzile, N. (2009). Antimony in the environment: Knowns and unknowns. Environmental Chemistry, 6, 95–105.
Foyer, C. H., & Noctor, G. (2005). Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment, 28, 1056–1071.
Foyer, C. H., & Noctor, G. (2011). Ascorbate and glutathione: the heart of the redox hub. Plant Physiology, 155, 2–18.
Fu, J., & Huang, B. (2001). Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environmental and Experimental Botany, 45(2), 105–114.
Gálvez, M., Martín-Cordero, C., Houghton, P. J., & Ayuso, M. J. (2008). Antioxidant activity of methanol extracts obtained from Plantago species. Journal of Agricultural and Food Chemistry, 53, 1927–1933.
Garrido, I., García-Sánchez, M., Casimiro, I., Casero, J., García-Romera, I., Ocampo, J. A., et al. (2012). Oxidative stress induced in sunflower seedling roots by aqueous dry olive-mill residues. PLoS ONE, 7(9), e46137.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plantas. Plant Physiology and Biochemistry, 40, 909–930.
Hawrylak-Nowak, B., Dresler, S., & Wójcik, M. (2014). Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Scientia Horticulturae, 172, 10–18.
He, M., Wang, X., Wu, F., & Fu, Z. (2012). Antimony pollution in China. Science of the Total Environment, 421–422, 41–50.
Herath, I., Vithanage, M., & Bundschuh, J. (2017). Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environmental Pollution, 223, 545–559.
Hossain, M.A., Piyatida, P., Teixeira da Silva, J.A., & Fujita, M. (2012). Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. Journal of Botany, ID 872875.
Husson, O., Husson, B., Brunet, A., Babre, D., Alary, K., Sarthou, J. P., et al. (2016). Practical improvements in soil redox potential (Eh) measurement for characterisation of soil properties. Application for comparison of conventional and conservation agriculture cropping systems. Analytica Chimica Acta, 906, 98–109.
Ji, Y., Sarret, G., Schulin, R., & Tandy, S. (2017). Fate and chemical speciation of antimony (Sb) during uptake, translocation and storage by rye grass using XANES spectroscopy. Environmental Pollution, 231, 1322–1329.
Jin, Y. H., Tao, D. L., Hao, Z. Q., Ye, J., Du, Y. J., Liu, H. L., et al. (2003). Enviromental stress and redox status of ascorbate. Acta Botanica Sinica, 45, 795–801.
Jozefczak, M., Remans, T., Vangronsveld, J., & Cuypers, A. (2012). Glutathione is a key player in metal induced oxidative stress defenses. International Journal of Molecular Sciences, 3, 3145–3175.
Kapoor, D., Sharma, R., Handa, N., Kaur, H., Rattan, A., Yadav, P., et al. (2015). Redox homeostasis in plants under abiotic stress: Role of electron carriers, energy metabolism mediators and proteinaceous thiols. Frontiers in Environmental Science. https://doi.org/10.3389/fenvs.2015.00013.
Karacan, M. S., Rodionova, M. V., Tunç, T., Venedik, K. B., Mamaş, S., Shitov, A. V., et al. (2016). Characterization of nineteen antimony(III) complexes as potent inhibitors of photosystem II, carbonic anhydrase, and glutathione reductase. Photosynthesis Research, 130, 1–16. https://doi.org/10.1007/s11120-016-0236-z.
Kim, D., Jeong, S. W., & Leo, C. Y. (2003). Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry, 81, 321–326.
Korkina, L. G. (2007). Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell and Molecular Biology, 53(1), 15–25.
Lehotai, N., Kolbert, Z., Petó, A., Feigl, G., Ördög, O., Kumar, D., et al. (2012). Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. Journal of Experimental Botany, 65, 5677–5687.
Michalak, A. (2006). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal of Environmental Studies, 15(4), 523–530.
Misra, H. P., & Fridovich, I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry, 247(10), 3170–3175.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405–410.
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). The reactive oxygen gene network in plants. Trends in Plant Science, 9, 490–498.
Murciego, A., García-Sánchez, A., Rodríguez-González, M. A., Pinilla, E., Toro, C., Cabezas, J., et al. (2007). Antimony distribution and mobility in topsoils and plants (Cytisus striatus, Cistus ladanifer and Dittrichia viscosa) from polluted Sb-mining areas in Extremadura (Spain). Environmental Pollution, 145, 15–21.
Ngo, T. T., & Lenhoff, H. M. (1980). A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Analytical Biochemistry, 195, 389–397.
Ning, Z., Xiao, T., & Xiao, E. (2015). Antimony in the soil-plant system in an Sb mining/smelting area of Southwest China. International Journal of Phytoremediation, 17, 1081–1089.
Okkenhaug, G., Zhu, Y.-G., Luo, L., Lei, M., Li, X., & Mulder, J. (2011). Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environmental Pollution, 159, 2427–2434.
Ortega, A., Garrido, I., Casimiro, I., & Espinosa, F. (2017). Effects of antimony on redox activities and antioxidant defence systems in sunflower (Helianthus annuus L.) plants. PLoS ONE, 12(9), e0183991.
Oxborough, K., & Baker, N. R. (1997). Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and Fv′/Fm′, without measuring Fo´. Photosynthesis Research, 54, 135–142.
Pan, X., Zhang, D., Chen, X., Bao, A., & Li, L. (2011). Antimony accumulation, growth performance, antioxidant defense system and photosynthesis of Zea mays, in response to antimony pollution in soil. Water, Air, and Soil pollution, 215, 517–523.
Pérez-Sirvent, C., Martínez-Sánchez, M. J., Martínez-López, S., Bech, J., & Bolan, N. (2012). Distribution and bioaccumulation of arsenic and antimony in Dittrichia viscosa growing in mining-affected semiarid soils in southeast Spain. Journal of Geochemical Exploration, 123, 128–135.
Pierart, A., Shahid, M., Séjalon-Delmas, N., Dumat, C. (2015). Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. Journal of Hazardous Materials, 289, 219e234.
Rauret, G., Lopez-Sanchez, J. F., Sahuquillo, A., Barohona, E., Lachica, M., Ure, A. M., et al. (2000). Application of modified BCR sequential extraction (three step) procedure for the determination of extractable trace metal content in a sewage sludge amended soil reference material (CRM 483), complemented by a three year stability study of acetic acid and EDTA extractable metal content. Journal of Environmental Monitoring, 2, 228–233.
Sakihama, Y., Cohen, M. F., Grace, S. C., & Yamasaki, H. (2002). Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology, 177, 67–80.
Samanta, A., Das, G., Das, S.K. (2011). Roles of flavonoids in plants. International Journal of Pharmaceutical Science and Technology, 6 (1).
Scandalios, J.G. (2005). Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Brazilian Journal of Medical and Biological Research, 38, 995e1014.
Serafimovska, J. M., Arpadjan, S., Stafilov, T., & Tsckova, K. (2013). Study of the antimony species distribution in industrially contaminated soils. Journal of Soils and Sediments, 13(2), 294–303.
Shantangeeva, I., Bali, R., & Harris, A. (2011). Bioavailability and toxicity of antimony. Journal of Geochemical Exploration, 110, 40–45.
Sharma, P., Jha, A.B., Dubey, R.S., Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012: ID 217037.
Singh, A., Agrawai, M., & Marshall, F. M. (2010). The role of organic vs inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecological Engineering, 36, 1733–1740.
Singh, V. P., Singh, S., Kumar, J., & Prasad, S. M. (2015). Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma, 252(3), 1217–1229.
Singleton, V. L., Salgues, M., Zaya, J., & Troudsale, E. (1985). Caftaric acid disappearance and conversion to products of enzymatic oxidation in grape must and wine. American Journal of Enology and Viticulture, 36, 50–56.
Spuller, C., Weigand, H., & Marb, C. (2007). Trace metal stabilization in a shooting range soil: Mobility and phytotoxicity. Journal of Hazardous Materials, 141, 378–387.
Srivastava, S., Mishra, S., Tripathi, R.D., Dwivedi, S., Gupta, K. (2006). Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hybrilla verticillarta (L.f.) Royle. Aquatic Toxicology, 80 (4), 405-415.
Srivastava, R. K., Pandey, P., Rajpoot, R., Rani, A., & Dubey, R. S. (2014). Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma, 251, 1047–1065.
Stolbovoy, V., Montanarella, L., Filippi, N., Jones, A., Gallego, J., & Grassi, G. (2007). Soil sampling protocol to certify the changes of organic carbon stock in mineral soil of the European Union. Version 2. EUR 21576 EN/2. Luxembourg, 2007, Office for Official Publications of the European Communities.
Sun, H., Yan, S. C., & Cheng, W. S. (2000). Interaction of antimony tartrate with the tripeptide glutathione. Implication for its mode of action. European Journal oif Biochemistry, 267, 5450–5457.
Thipyapong, P., Hunt, M. D., & Steffens, J. C. (1995). Systemic wound induction of potato (Solanum tuberosum) polyphenol oxidase. Phytochemistry, 40, 673–676.
Volkova, L. A., Urmatseva, V. V., & Burgutin, A. B. (2014). Stress-protective effect of phenylpropanoid complex on potato plants in vitro. Russian Journal of Plant Physiology, 61, 255–261.
Waszczak, C., Carmody, M., & Kangasjärvi, J. (2018). Reactive oxygen species in plant signaling. Annual Review of Plant Biology, 69, 209–236.
Wellburn, A. R. (1994). The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144(3), 307–313.
WHO. (2003). Antimony in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva: World Health Organization (WHO/SDE/WSH/03.04/74).
Wysocki, R., Clemens, S., Augustyniak, D., Golik, P., Maciaszczyk, E., Tamás, M. J., et al. (2003). Metalloid tolerance base don phytochelatins is not functionally equivalent to the arsenite transporter Acr3p. Biochemical and Biophysical Research Communications, 304, 293–300.
Xue, L., Ren, H., Li, S., Gao, M., Shi, S., Chang, E., et al. (2015). Comparative proteomic analysis in Miscanthus sinensis exposed to antimony stress. Environmental Pollution, 201, 150–160.
Yadav, S. K. (2010). Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metals stress tolerance of plants. South African Journal of Botany, 76, 167–179.
Yamasaki, H., Sakihama, Y., & Ikehara, N. (1997). Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiology, 115, 1405–1412.
Zeng, B., Wang, F. J., Zhu, C., & Sun, Z. X. (2008). Effect of ascorbate-glutathione (AsA-GSH) cycle on Hg2+-tolerance in rice mutant. Acta Agronomica Sinica, 34(5), 823–830.
Zhou, X., Sun, C., Zhu, P., & Liu, F. (2018). Effects of antimony stress on photosynthesis and growth of Acorus calamus. Frontiers in Plant Science, 9, 579.
Acknowledgements
This study was made possible thanks to La Junta de Extremadura/FEDER for Research Project IB16078 and support given to Research Group FBCMP (GR18168).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garrido, I., Ortega, A., Hernández, M. et al. Effect of antimony in soils of an Sb mine on the photosynthetic pigments and antioxidant system of Dittrichia viscosa leaves. Environ Geochem Health 43, 1367–1383 (2021). https://doi.org/10.1007/s10653-020-00616-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00616-0