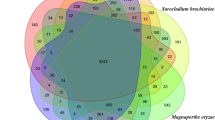
Abstract
Armillaria species (Basidiomycota, Physalacriaceae) are well known as plant pathogens related to serious root rot disease on various trees in forests and plantations. Interestingly, some Armillaria species are essential symbionts of the rare Chinese medicinal herb Gastrodia elata, a rootless and leafless orchid used for over 2000 years. In this work, an 87.3-M draft genome of Armillaria gallica 012m strain, which was symbiotic with G. elata, was assembled. The genome includes approximately 23.6% repetitive sequences and encodes 26,261 predicted genes. In comparison with other four genomes of Armillaria, the following gene families related to pathogenicity/saprophytic phase, including cytochrome P450 monooxygenases, carbohydrate-active enzyme AA3, and hydrophobins, were significantly contracted in A. gallica 012m. These characteristics may be beneficial for G. elata to get less injuries. The genome-guided analysis of differential expression between rhizomorph (RH) and vegetative mycelium (VM) showed that a total of 2549 genes were differentially expressed, including 632 downregulated genes and 1917 upregulated genes. In the RH, most differentially expressed genes (DEGs) related to pathogenicity were significantly upregulated. To further elucidate gene function, Gene Ontology enrichment analysis showed that the upregulated DEGs significantly grouped into monooxygenase activity, hydrolase activity, glucosidase activity, extracellular region, fungal cell wall, response to xenobiotic stimulus, response to toxic substance, etc. These phenomena indicate that RH had better infection ability than VM. The infection ability of RH may be beneficial for G. elata to obtain nutrition, because the rhizomorph constantly infected the nutritional stems of G. elata and formed the hyphae that can be digested by G. elata. These results clarified the characteristics of A. gallica 012m and the reason why the strain 012m can establish a symbiotic relationship with G. elata in some extent from the perspective of genomics.






Similar content being viewed by others
References
Xu J, Guo S (2000) Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chin Med J 113(8):686–692
Li HB, Chen F (2004) Preparative isolation and purification of gastrodin from the Chinese medicinal plant Gastrodia elata by high-speed counter-current chromatography. J Chromatogr A 1052(1–2):229–232
Zhan HD, Zhou HY, Sui YP, Du XL, Wang WH, Dai L, Sui F, Huo HR, Jiang TL (2016) The rhizome of Gastrodia elata Blume-an ethnopharmacological review. J Ethnopharmacol 189:361–385. https://doi.org/10.1016/j.jep.2016.06.057
Hayashi J, Sekine T, Deguchi S, Lin Q, Horie S, Tsuchiya S, Yano S, Watanabe K, Ikegami F (2002) Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry 59(5):513–519
Zeng X, Li Y, Ling H, Chen J, Guo S (2018) Revealing proteins associated with symbiotic germination of Gastrodia elata by proteomic analysis. Bot Stud 59(1):8. https://doi.org/10.1186/s40529-018-0224-z
Kim YI, Chang KJ, Ka KH, Hur H, Hong IP, Shim JO, Lee TS, Lee JY, Lee MW (2006) Seed germination of Gastrodia elata using symbiotic fungi, Mycena osmundicola. Mycobiology 34(2):79–82. https://doi.org/10.4489/MYCO.2006.34.2.079
Xu JT (1989) Studies on the life cycle of Gastrodia elata. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 11(4):237–241
Shaw CG, Kile GA, United States. Forest Service. (1991) Armillaria root disease. Agriculture handbook, vol no 691. Forest Service, U.S. Dept. of Agriculture, Washington, D.C.
Baumgartner K, Coetzee MP, Hoffmeister D (2011) Secrets of the subterranean pathosystem of Armillaria. Mol Plant Pathol 12(6):515–534. https://doi.org/10.1111/j.1364-3703.2010.00693.x
Sipos G, Anderson JB, Nagy LG (2018) Armillaria. Curr Biol 28(7):R297–R298. https://doi.org/10.1016/j.cub.2018.01.026
SUN S-q, CHEN G-h (2003) The influences of different armillaria on the yield of Gastrodia and the content of gastrodin [J]. Shandong Science 2
Wang Q, Guo S, Guan F (1994) Studies on influences of Armillaria mellea strains from different sources on yield of Gastrodia elata. Chinese Traditional and Herbal Drugs (09)
Tsai CC, Wu KM, Chiang TY, Huang CY, Chou CH, Li SJ, Chiang YC (2016) Comparative transcriptome analysis of Gastrodia elata (Orchidaceae) in response to fungus symbiosis to identify gastrodin biosynthesis-related genes. BMC Genomics 17:212. https://doi.org/10.1186/s12864-016-2508-6
Yuan Y, Jin X, Liu J, Zhao X, Zhou J, Wang X, Wang D, Lai C, Xu W, Huang J, Zha L, Liu D, Ma X, Wang L, Zhou M, Jiang Z, Meng H, Peng H, Liang Y, Li R, Jiang C, Zhao Y, Nan T, Jin Y, Zhan Z, Yang J, Jiang W, Huang L (2018) The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat Commun 9(1):1615. https://doi.org/10.1038/s41467-018-03423-5
Gregory S (1985) The use of potato tubers in pathogenicity studies of Armillaria isolates. Plant Pathol 34(1):41–48
Morrison D (2004) Rhizomorph growth habit, saprophytic ability and virulence of 15 Armillaria species. For Pathol 34(1):15–26
Redfern D (1975) Influence of food base on rhizomorph growth and pathogenicity of Armillaria mellea isolates. In: Biology and Control of Soil Borne Plant Pathogens International Symposium
Rishbeth J (1982) Species of Armillaria in southern England. Plant Pathol 31(1):9–17
Guo T, Wang HC, Xue WQ, Zhao J, Yang ZL (2016) Phylogenetic analyses of Armillaria reveal at least 15 phylogenetic lineages in China, seven of which are associated with cultivated Gastrodia elata. PLoS One 11(5):e0154794. https://doi.org/10.1371/journal.pone.0154794
Sipos G, Prasanna AN, Walter MC, O’Connor E, Bálint B, Krizsán K, Kiss B, Hess J, Varga T, Slot J (2017) Genome expansion and lineage-specific genetic innovations in the forest pathogenic fungi Armillaria. Nature Ecology & Evolution 1(12):1931–1941
Collins C, Keane TM, Turner DJ, O’Keeffe G, Fitzpatrick DA, Doyle S (2013) Genomic and proteomic dissection of the ubiquitous plant pathogen, Armillaria mellea: toward a new infection model system. J Proteome Res 12(6):2552–2570. https://doi.org/10.1021/pr301131t
Wingfield BD, Ambler JM, Coetzee MP, de Beer ZW, Duong TA, Joubert F, Hammerbacher A, McTaggart AR, Naidoo K, Nguyen HD, Ponomareva E, Santana QS, Seifert KA, Steenkamp ET, Trollip C, van der Nest MA, Visagie CM, Wilken PM, Wingfield MJ, Yilmaz N (2016) IMA Genome-F 6: draft genome sequences of Armillaria fuscipes, Ceratocystiopsis minuta, Ceratocystis adiposa, Endoconidiophora laricicola, E. polonica and Penicillium freii DAOMC 242723. IMA Fungus 7(1):217–227. https://doi.org/10.5598/imafungus.2016.07.01.11
Heinzelmann R, Dutech C, Tsykun T, Labbé F, Soularue JP, Prospero S (2018) Latest advances and future perspectives in Armillaria research
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Gnerre S, Maccallum I, Przybylski D, Ribeiro FJ, Burton JN, Walker BJ, Sharpe T, Hall G, Shea TP, Sykes S, Berlin AM, Aird D, Costello M, Daza R, Williams L, Nicol R, Gnirke A, Nusbaum C, Lander ES, Jaffe DB (2011) High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc Natl Acad Sci U S A 108(4):1513–1518. https://doi.org/10.1073/pnas.1017351108
Salmela L, Rivals E (2014) LoRDEC: accurate and efficient long read error correction. Bioinformatics 30(24):3506–3514. https://doi.org/10.1093/bioinformatics/btu538
Xiao C-L, Chen Y, Xie S-Q, Chen K-N, Wang Y, Han Y, Luo F, Xie Z (2017) MECAT: fast mapping, error correction, and de novo assembly for single-molecule sequencing reads. nature methods 14 (11):1072
Roach MJ, Schmidt SA, Borneman AR (2018) Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC bioinformatics 19(1):460
Lam KK, LaButti K, Khalak A, Tse D (2015) FinisherSC: a repeat-aware tool for upgrading de novo assembly using long reads. Bioinformatics 31(19):3207–3209. https://doi.org/10.1093/bioinformatics/btv280
Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25(5):955–964
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW (2007) RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35(9):3100–3108. https://doi.org/10.1093/nar/gkm160
Bao W, Kojima KK, Kohany O (2015) Repbase Update, a database of repetitive elements in eukaryotic genomes. Mob DNA 6:11. https://doi.org/10.1186/s13100-015-0041-9
Floudas D, Held BW, Riley R, Nagy LG, Koehler G, Ransdell AS, Younus H, Chow J, Chiniquy J, Lipzen A (2015) Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genet Biol 76:78–92
Wawrzyn GT, Quin MB, Choudhary S, López-Gallego F, Schmidt-Dannert C (2012) Draft genome of Omphalotus olearius provides a predictive framework for sesquiterpenoid natural product biosynthesis in Basidiomycota. Chem Biol 19(6):772–783
Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A (2015) Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47(4):410–415
Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, Riley R, Salamov A, Zhao X, Korzeniewski F, Smirnova T, Nordberg H, Dubchak I, Shabalov I (2014) MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res 42(Database issue):D699–D704. https://doi.org/10.1093/nar/gkt1183
Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM (2015) BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31(19):3210–3212. https://doi.org/10.1093/bioinformatics/btv351
Buchfink B, Xie C, Huson DH (2015) Fast and sensitive protein alignment using DIAMOND. Nat Methods 12(1):59–60. https://doi.org/10.1038/nmeth.3176
Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S (2014) InterProScan 5: genome-scale protein function classification. Bioinformatics 30(9):1236–1240. https://doi.org/10.1093/bioinformatics/btu031
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21(18):3674–3676. https://doi.org/10.1093/bioinformatics/bti610
Ye J, Zhang Y, Cui H, Liu J, Wu Y, Cheng Y, Xu H, Huang X, Li S, Zhou A, Zhang X, Bolund L, Chen Q, Wang J, Yang H, Fang L, Shi C (2018) WEGO 2.0: a web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res 46(W1):W71–W75. https://doi.org/10.1093/nar/gky400
Sipos G, Prasanna AN, Walter MC, O’Connor E, Balint B, Krizsan K, Kiss B, Hess J, Varga T, Slot J, Riley R, Boka B, Rigling D, Barry K, Lee J, Mihaltcheva S, LaButti K, Lipzen A, Waldron R, Moloney NM, Sperisen C, Kredics L, Vagvolgyi C, Patrignani A, Fitzpatrick D, Nagy I, Doyle S, Anderson JB, Grigoriev IV, Guldener U, Munsterkotter M, Nagy LG (2017) Genome expansion and lineage-specific genetic innovations in the forest pathogenic fungi Armillaria. Nat Ecol Evol 1(12):1931–1941. https://doi.org/10.1038/s41559-017-0347-8
Ohm RA, De Jong JF, Lugones LG, Aerts A, Kothe E, Stajich JE, De Vries RP, Record E, Levasseur A, Baker SE (2010) Genome sequence of the model mushroom Schizophyllum commune. Nat Biotechnol 28(9):957–963
Morin E, Kohler A, Baker AR, Foulongne-Oriol M, Lombard V, Nagye LG, Ohm RA, Patyshakuliyeva A, Brun A, Aerts AL (2012) Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc Natl Acad Sci 109(43):17501–17506
Varga T, Krizsán K, Földi C, Dima B, Sánchez-García M, Sánchez-Ramírez S, Szöllősi GJ, Szarkándi JG, Papp V, Albert L (2019) Megaphylogeny resolves global patterns of mushroom evolution. Nature Ecology & Evolution 3(4):668–678
Martin F, Aerts A, Ahrén D, Brun A, Danchin E, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452(7183):88–92
Stajich JE, Wilke SK, Ahrén D, Au CH, Birren BW, Borodovsky M, Burns C, Canbäck B, Casselton LA, Cheng C (2010) Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proc Natl Acad Sci 107(26):11889–11894
Riley R, Salamov AA, Brown DW, Nagy LG, Floudas D, Held BW, Levasseur A, Lombard V, Morin E, Otillar R (2014) Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci 111(27):9923–9928
Emms DM, Kelly S (2018) OrthoFinder2: fast and accurate phylogenomic orthology analysis from gene sequences. BioRxiv:466201
Laetsch DR, Blaxter ML (2017) KinFin: software for taxon-aware analysis of clustered protein sequences. G3: Genes, Genomes, Genetics 7 (10):3349–3357
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Talavera G, Castresana J (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56(4):564–577. https://doi.org/10.1080/10635150701472164
Darriba D, Taboada GL, Doallo R, Posada D (2011) ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27(8):1164–1165. https://doi.org/10.1093/bioinformatics/btr088
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2014) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772
Sanderson MJ (2003) r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19(2):301–302
Sanderson MJ (2002) Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol Biol Evol 19(1):101–109. https://doi.org/10.1093/oxfordjournals.molbev.a003974
Nash SG (2000) A survey of truncated-Newton methods. J Comput Appl Math 124(1–2):45–59
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336(6089):1715–1719
Chen L, Gong Y, Cai Y, Liu W, Zhou Y, Xiao Y, Xu Z, Liu Y, Lei X, Wang G (2016) Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into lignocellulose degradation. PLoS One 11(8):e0160336
Letunic I, Bork P (2019) Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259
Tijl DB, Nello C, Demuth JP, Hahn MW (2006) CAFE: a computational tool for the study of gene family evolution. Bioinformatics 22(10):1269–1271
Meinhardt LW, Costa GGL, Thomazella DP, Teixeira PJP, Carazzolle MF, Schuster SC, Carlson JE, Guiltinan MJ, Mieczkowski P, Farmer A (2014) Genome and secretome analysis of the hemibiotrophic fungal pathogen, Moniliophthora roreri, which causes frosty pod rot disease of cacao: mechanisms of the biotrophic and necrotrophic phases. BMC Genomics 15(1):164
Mondego JM, Carazzolle MF, Costa GG, Formighieri EF, Parizzi LP, Rincones J, Cotomacci C, Carraro DM, Cunha AF, Carrer H (2008) A genome survey of Moniliophthora perniciosa gives new insights into witches’ broom disease of cacao. BMC Genomics 9(1):548
Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y (2012) dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res 40 (Web Server issue):W445-451. doi:https://doi.org/10.1093/nar/gks479
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
Chen C, Chen H, He Y, Xia R (2018) TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv:289660
Sützl L, Laurent CV, Abrera AT, Schütz G, Ludwig R, Haltrich D (2018) Multiplicity of enzymatic functions in the CAZy AA3 family. Appl Microbiol Biotechnol 102(6):2477–2492
Leonowicz A, Rogalski J, Jaszek M, Luterek J, Wojtas-Wasilewska M, Malarczyk E, Ginalska G, Fink-Boots M, Cho N-S (1999) Cooperation of fungal laccase and glucose 1-oxidase in transformation of Björkman lignin and some phenolic compounds. Holzforschung 53(4):376–380
Carro J, Serrano A, Ferreira P, Martínez AT (2016) Fungal aryl-alcohol oxidase in lignocellulose degradation and bioconversion. In: Microbial enzymes in bioconversions of biomass. Springer, pp 301–322
Kgosiemang IKR, Syed K, Mashele SS (2014) Comparative genomics and evolutionary analysis of cytochrome P450 monooxygenases in fungal subphylum Saccharomycotina
Park J, Lee S, Choi J, Ahn K, Park B, Park J, Kang S, Lee Y-H (2008) Fungal cytochrome P450 database. BMC Genomics 9(1):402
van Gorcom RF, van den Hondel CA, Punt PJ (1998) Cytochrome P450 enzyme systems in fungi. Fungal Genet Biol 23(1):1–17
Wessels J (1994) Developmental regulation of fungal cell wall formation. Annu Rev Phytopathol 32(1):413–437
Wessels J (2000) Hydrophobins, unique fungal proteins. Mycologist 14(4):153–159
Wösten HA (2001) Hydrophobins: multipurpose proteins. Annual Reviews in Microbiology 55(1):625–646
Wessels JG (1996) Hydrophobins: proteins that change the nature of the fungal surface. In: Advances in microbial physiology, vol 38. Elsevier, pp 1-45
Wösten H, Schuren F, Wessels J (1994) Interfacial self-assembly of a hydrophobin into an amphipathic protein membrane mediates fungal attachment to hydrophobic surfaces. EMBO J 13(24):5848–5854
Wessels JG, De Vries OM, Asgeirsdottir SA, Schuren FH (1991) Hydrophobin genes involved in formation of aerial hyphae and fruit bodies in Schizophyllum. Plant Cell 3(8):793–799
Kim S, Ahn IP, Rho HS, Lee YH (2005) MHP1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol Microbiol 57(5):1224–1237
Klimes A, Dobinson KF (2006) A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet Biol 43(4):283–294
Talbot NJ, Kershaw MJ, Wakley GE, De Vries OM, Wessels JG, Hamer JE (1996) MPG1 encodes a fungal hydrophobin involved in surface interactions during infection-related development of Magnaporthe grisea. Plant Cell 8(6):985–999
Whiteford JR, Spanu PD (2001) The hydrophobin HCf-1 of Cladosporium fulvum is required for efficient water-mediated dispersal of conidia. Fungal Genet Biol 32(3):159–168
Temple B, Horgen PA (2000) Biological roles for cerato-ulmin, a hydrophobin secreted by the elm pathogens, Ophiostoma ulmi and O. novo-ulmi. Mycologia:1–9
Xu J, Fan L (2001) Cytodifferentiation of the seeds (protocorms) and vegetative propagation corms colonized by mycorrhizal fungi. Acta Bot Sin 43(10):1003–1010
Jin-tang X (2001) The changes of cell structure in the courses of Armillaria mellea penetrating the nutritional stems of Gastrodia elata. Acta-Academiae Medicinae Sinicae 23(2):150–153
Funding
This research was financially supported by the National Natural Science Foundation of China (Nos. 81860624 and 31760096) and Yunnan Innovative Research Team for Discovery and Comprehensive Utilization of Functional Small Molecules in Medicinal Plants.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Rodrigo Galhardo.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhan, M., Tian, M., Wang, W. et al. Draft genomic sequence of Armillaria gallica 012m: insights into its symbiotic relationship with Gastrodia elata. Braz J Microbiol 51, 1539–1552 (2020). https://doi.org/10.1007/s42770-020-00317-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00317-x