-
PDF
- Split View
-
Views
-
Cite
Cite
Yu-yong Liang, Mei Luo, Xiao-gang Fu, Li-xia Zheng, Hong-yi Wei, Mating Disruption of Chilo suppressalis From Sex Pheromone of Another Pyralid Rice Pest Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), Journal of Insect Science, Volume 20, Issue 3, May 2020, 19, https://doi.org/10.1093/jisesa/ieaa050
Close - Share Icon Share
Abstract
The rice stem borer, Chilo suppressalis (Walker), and the rice leaf folder, Cnaphalocrocis medinalis Guenée, are two of the most destructive lepidopteran pests in rice. Since these two pyralid insects overlap in their occurrence in rice paddy fields, farmers prefer to set their pheromone-baited traps together in the rice fields for their monitoring. However, our field observation demonstrated that no male adult of C. suppressalis was captured in traps baited with commercial sex pheromone of C. suppressalis (CCS) combined with commercial sex pheromone of C. medinalis (CCM). To confirm that the C. medinalis sex pheromone component(s) interfere with the attraction of males of the rice stem borers to their conspecific females, single components of C. medinalis sex pheromone combined with CCS in traps were tested in the laboratory and rice paddy field. The results revealed that the two alcohol components in CCM, i.e., (Z)-11-octadecen-1-ol (Z11-18: OH) and (Z)-13-octadecen-1-ol (Z13-18: OH) may cause a significant reduction in capturing C. suppressalis males caused by CCS. We recommend against using these sex pheromones together in the field and suggest that Z11-18: OH and Z13-18: OH could be potential inhibitors or antagonists of C. suppressalis sex pheromone to control the rice stem borer.
The rice stem borer, Chilo suppressalis (Walker) and the rice leaf folder, Cnaphalocrocis medinalis Guenée, are two of the most harmful lepidopteran rice pests throughout China and other Asian countries (Su et al. 2014, Luo et al. 2019). These two pests are destructive agricultural pests distributed in all over China (Sheng et al. 2003, Bao et al. 2015) and cause severe damage to rice at the growth stage, which results in wide and extensive damages in rice and sever economic loss (Sheng et al. 2003, Liu et al. 2008). In recent years, application of chemical insecticides has been utilized as an efficient method to control C. suppressalis and C. medinalis in China (Huang et al. 2011, Zheng et al. 2011). For example, chlorantraniliprole, chlorpyrifos and deltamethrin have been suggested to be useful in controlling these two pests (Chen and Klein 2012, Yang et al. 2018). However, the overuse of insecticides may cause severe insecticide resistance and serious environmental problems (Chen and Klein 2012, Fu et al. 2018, Pu et al. 2020). Therefore, biological control has been recommended as one of the most important alternative strategies to suppress the outbreaks of these rice pests (Lou et al. 2014).
Sex pheromone application is becoming a valuable and efficient strategy of biotechnological control to suppress lepidopteran pests (Chen et al. 2014). For example, pheromone-baited traps have been widely used in male flight monitoring, population forecasting, mass trapping, and mating disruption to control a lot of lepidopteran pest species (Campion and Nesbitt 1983, Witzgall et al. 2010, Chen et al. 2014). Compared with conventional methods, the pheromone-based control methods have the advantages of species-specific and strong bioactivities, which leads to small amounts are required and negligible toxic effects on plants and nontarget animals (Campion and Nesbitt 1983).
In most lepidopteran insects, sex pheromones are generally secreted by the females for attracting the males for copulation (Raina 1989). The C. suppressalis sex pheromone was first identified by Nesbitt et al. (1975) as a two-component blend of (Z)-11-hexadecenal (Z11-16:Ald) and (Z)-13-octadecenal (Z13-18:Ald). In 1983, an additional compound, (Z)-9-hexadecenal (Z9-16:Ald), was identified (Tatsuki et al. 1983). The ratio of these three-components (Z11-16:Ald, Z13-18:Ald, and Z9-16:Ald) in C. suppressalis is 48:6:5, which was used in the production of the standard lure of C. suppressalis (Tatsuki 1990, Cork 2004). The C. medinalis sex pheromone consists of four components, (Z)-11-octadecenal (Z11-18:Ald), (Z)-13-octadecenal (Z13-18:Ald), (Z)-11-octadecen-1-ol (Z11-18:OH) and (Z)-13-octadecen-1-ol (Z13-18:OH) in a ratio of 11:100:24:36 (Kawazu et al. 2000). Since then, the sex pheromones of C. suppressalis and C. medinalis have been widely applied in rice paddy fields and have become an important part of integrated pest management (IPM) programs (Kawazu et al. 2004, 2005; Byers 2007; Litsinger 2009; Cho et al. 2013; Chen et al. 2014).
Chilo suppressalis has four generations per year in most parts of Jiangxi province, China. In addition, the distribution and emergence periods of C. suppressalis and C. medinalis overlap extensively (Luo et al. 2019). For this reason, the two sex pheromones of the two species may be used at the same time by farmers to control both pests. However, previous studies have reported that mixing sex pheromones of two pest species resulted in the interference of captures (Haynes et al. 2002, Gemeno et al. 2006). We hypothesize that mixing of the sex pheromones of C. suppressalis and C. medinalis might interfere with the captures of these two pests, which have not been investigated yet. Therefore, in this study, we aimed to determine whether mixing the sex pheromones of C. suppressalis and C. medinalis has negative effects in the IPM of these two pests. We also wanted to determine the underlying mechanism of these negative effects on pest control from the mixing of the two sex pheromones. This study is very important for guiding farmers in the intensive application of various sex pheromones for pest control.
Materials and Methods
Insects
The C. suppressalis laboratory colony was collected from Shanggao county, Jiangxi province, eastern China (28°19′10.87″ N, 115°04′53.34″ E). Naturally overwintering larvae were collected from paddy fields in late March 2012 and then transferred to cylindrical glass jars (10.0 cm in height and 12.0 cm in diameter) with several rice stems. In total, 20–30 C. suppressalis individuals were reared per jar. The rice stems were changed every two days. Pupae were collected and transferred to 24-well plates for individual emergence. Newly emerged female and male adults were maintained separately in transparent plastic bags (30.0-cm length and 20.0-cm width) with a 10% sucrose solution. The insects were maintained in a growth room under a photo regime of 14:10 (L:D) h at 25 ± 1°C and 85 ± 5% RH.
Chemicals
The lure of commercial sex pheromones of C. suppressalis (CCS) contains Z11-16:Ald, Z13-18:Ald and Z9-16:Ald. The lure of commercial sex pheromones of C. medinalis (CCM) includes Z11-18:Ald, Z13-18:Ald, Z11-18:OH, and Z13-18:OH. Each lure contains 0.5–2.0 mg of each component. The load of lures is green rubber septa. The lures of CCS or CCM were purchased from Pherobio Technology Co., Ltd (Beijing, China). The synthetic chemicals Z11-16:Ald, Z13-18:Ald, Z9-16:Ald, Z11-18:Ald, Z11-18:OH, and Z13-18:OH with >99% purity were obtained from Sigma Chemical Co. (St. Louis, MO). The pure synthetic chemicals were diluted, then rubber septa were impregnated with the diluted solution. Our previous study has tested the response of male C. suppressalis to sex pheromone components of C. medinalis with different concentrations and determined that the optimal concentration of CS (three components of C. suppressalis sex pheromone (Z11-16:Ald, Z13-18:Ald, Z9-16:Ald = 48:6:5) is 1 μg/μl. Therefore, rubber septa impregnated with 200 µl (1 µg/µl) of the synthetic chemicals. The different lures with synthetic chemicals were used in the laboratory tests and field experiments.
Experimental Devices
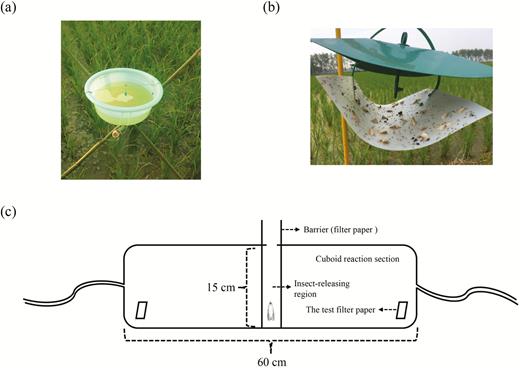
In 2012, we used a simple trap (trap 1; Fig. 1a) in the field experiment, which was generally used by local famers. The trap 1 was custom-made, which consisted of a basin (diameter: 25.0 cm and depth: 10.0 cm) and a triangular bracket. Two drainage holes were made 6.0–7.0 cm away from the dish bottom, and the basin was filled with water until its level reached the drainage holes. 2.0 g washing powder was added to the water in the basin to prevent the pest escapes. The lures were always fixed 0.5–1.0 cm above the water. The basin was fixed on the bracket, and the height of the basin was always hanged to 10.0–20.0 cm higher than the rice plant.
The schematic diagrams of three experimental devices in this study. (a) trap 1, (b) trap 2, and (c) olfactometer.
In 2014, with the industrialization and promotion of traps, we chose a kind of more convenient trap (trap 2; Fig. 1b). The trap 2 was purchased from Pherobio Technology Co., Ltd (Beijing, China), which consisted of a ship-type trap and a trestle. The ship-type trap consisted of a sticky board, a bezel, and a jack device (four holes) for lures. Rubber septa with different sex pheromone components as lures were tested in the field experiments.
To test the avoidance effect of different components (two choices), we used a custom-made olfactometer (Fig. 1c). The olfactometer included a transparent plastic cuboid box as an experiment section (60.0-cm length, 15.0-cm width, and 15.0-cm height). The cuboid was averagely divided into three parts, an insect-releasing region in the middle and two testing regions at the two ends, respectively. Filter papers were placed in each of these regions. An exit opening was made on the back of the device. The olfactometer was kept on a table. Humidified and purified air were pumped into the device at 1.0 liter/min from both ends, respectively, by activated carbon filters. The lures were made of strips of filter paper (1.0 × 5.0 cm), which were loaded with either 100 µl (1 µg/µl) of different sex pheromone components or redistilled hexane (control).
Experimental Design
To determine whether mixture of sex pheromones of C. suppressalis and C. medinalis interferes with the capture of C. suppressalis and C. medinalis, we recorded the attraction of the traps baited with CCS and CCM to these two pests in the field (experiment 1; Table 1). The traps (trap 1) contained either the two sex pheromones or CCS only as the control. Three traps were set up to serve as replicates of each of the treatments. The test was conducted from July to September 2012 and trap captures were counted every day.
The experimental design
| Experiment . | Experimental purpose . | Experimental device . | Time . |
|---|---|---|---|
| Experiment 1 | To determine whether mixing the sex pheromones of C. suppressalis and C. medinalis interfere the capture of C. suppressalis and C. medinalis | Trap 1 | July to Sept. 2012 |
| Experiment 2 | To test the avoidance response of C. suppressalis male to sex pheromone components of C. medinalis | Olfactometer | July to Aug. 2013 |
| Experiment 3 | To examine the effects of the sex pheromone components of C. medinalis on C. suppressalis in the field | Trap 2 | June to Aug. 2014 |
| Experiment . | Experimental purpose . | Experimental device . | Time . |
|---|---|---|---|
| Experiment 1 | To determine whether mixing the sex pheromones of C. suppressalis and C. medinalis interfere the capture of C. suppressalis and C. medinalis | Trap 1 | July to Sept. 2012 |
| Experiment 2 | To test the avoidance response of C. suppressalis male to sex pheromone components of C. medinalis | Olfactometer | July to Aug. 2013 |
| Experiment 3 | To examine the effects of the sex pheromone components of C. medinalis on C. suppressalis in the field | Trap 2 | June to Aug. 2014 |
The experimental design
| Experiment . | Experimental purpose . | Experimental device . | Time . |
|---|---|---|---|
| Experiment 1 | To determine whether mixing the sex pheromones of C. suppressalis and C. medinalis interfere the capture of C. suppressalis and C. medinalis | Trap 1 | July to Sept. 2012 |
| Experiment 2 | To test the avoidance response of C. suppressalis male to sex pheromone components of C. medinalis | Olfactometer | July to Aug. 2013 |
| Experiment 3 | To examine the effects of the sex pheromone components of C. medinalis on C. suppressalis in the field | Trap 2 | June to Aug. 2014 |
| Experiment . | Experimental purpose . | Experimental device . | Time . |
|---|---|---|---|
| Experiment 1 | To determine whether mixing the sex pheromones of C. suppressalis and C. medinalis interfere the capture of C. suppressalis and C. medinalis | Trap 1 | July to Sept. 2012 |
| Experiment 2 | To test the avoidance response of C. suppressalis male to sex pheromone components of C. medinalis | Olfactometer | July to Aug. 2013 |
| Experiment 3 | To examine the effects of the sex pheromone components of C. medinalis on C. suppressalis in the field | Trap 2 | June to Aug. 2014 |
To test the avoidance response of male C. suppressalis to sex pheromone components of C. medinalis, we conducted a two-way choice bioassay on male C. suppressalis to different sex pheromone components of C. medinalis using a custom-made olfactometer (experiment 2; Table 1). For each bioassay, the males were introduced individually into the insect-releasing region of the device. The males were released after the barriers (two filter papers) were pulled out. When the male initiated flight and migrated to one of the testing ends (set over half of the choice chambers), the choice was recorded after it remained for at least 60.0 s in that testing end. If the male did not make a choice within 5.0 min after being released into the olfactometer, it was considered as a nonresponder. We rotated the olfactometer 180° to randomize any positional effects after five males have been tested. We cleaned the olfactometer with 95% ethyl alcohol after ten males have been tested. The test filter papers which contained different sex pheromone components were used as lures in this study. The lures were CCM; CCM + CCS; Z11-18:Ald; Z13-18:OH; Z11-18:OH; four-component blend of CCM, at a ratio of 100:36:11:24, of the Z13-18:Ald, Z13-18:OH, Z11-18:Ald, and Z11-18:OH (CM); the components of CCS and CCM, at a ratio of 140:117:15:36:11:24, of the Z11-16:Ald, Z13-18:Ald, Z9-16:Ald, Z13-18:OH, Z11-18:Ald, Z11-18:OH (CS+CM). We did not test the avoidance response of male C. suppressalis to Z13-18:Ald as it is also one of the sex pheromone components of C. suppressalis. Thirty males were tested in each treatment.
To examine the effects of the sex pheromone components of C. medinalis on C. suppressalis in the paddy field, we observed the attraction of male C. suppressalis to the traps baited with a combination of CCS and the components of CCM by the trap 2 (experiment 3; Table 1). The treatments were a combination of Z11-18:Ald, Z13-18:OH, and Z11-18:OH with CCS, respectively, and the control was CCS only. Three traps were set up to serve as replicates of each of the treatments. The test was conducted from June to August 2014 and trap captures were also counted every day.
Data Analysis
We compared the difference between treatments and control using SPSS 24.0 (SPSS Inc., Chicago, IL). Differences in olfactometer experiments were analyzed by a χ 2-test. The data normality was determined by Shapiro–Wilk test. The difference of number captured between two treatments was analyzed with the Mann–Whitney U-test. The significance level was set at 0.05.
Results
Mixing the Sex Pheromones of C. suppressalis and C. medinalis Interfered With the Capture of C. suppressalis
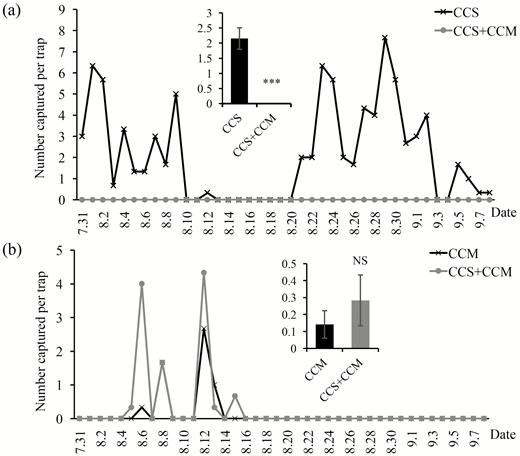
During the experimental period (July to September 2012), we found that no male C. suppressalis was caught in the traps baited with the combined CCS and CCM. However, the total captures by the traps baited with CCS only were 254 and the average trap was 2.12 males per trap per day, which were extremely significantly higher than those captured by the traps baited with the combined CCS and CCM (P < 0.001; Fig. 2a). Meanwhile, we caught 0.16 and 0.28 C. medinalis males per trap per day by the traps baited with CCM and the traps baited with the combined CCS and CCM, respectively, which showed no significance between these two treatments (P = 0.498; Fig. 2b). These results suggested that mixing the sex pheromones of C. suppressalis and C. medinalis interfered with the capture of C. suppressalis but not C. medinalis in the field.
Number of C. suppressalis (a) and C. medinalis (b) males captured by their commercial sex pheromone alone (CCS or CCM) (black) and combined with these two sex pheromone (CCS+CCM) (gray) in Jiangxi, China, from July to September 2012. We used trap 1 in the field experiment. CCS: commercial sex pheromones of C. suppressalis, CCM: commercial sex pheromones of C. medinalis, CCS+CCM: combination of commercial sex pheromones of C. suppressalis and C. medinalis. We compared the difference of capture of C. suppressalis and C. medinalis males between treatments and control treatment with Mann–Whitney U-test. ***P < 0.001, NS, no significance.
Z11-18:OH Was the Major Component of CCM That Inhibited the Attraction of Male C. suppressalis in Olfactory Behavior
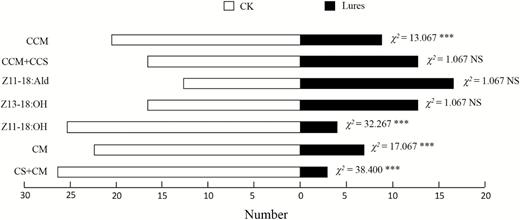
To probe the response of male C. suppressalis to sex pheromone components of C. medinalis, we tested the attractiveness of the seven lures to C. suppressalis males using a custom-made olfactometer (Fig. 3). We found that the lures of Z11-18:Ald (χ2= 1.067, P = 0.302), Z13-18:OH (χ2= 1.067, P = 0.302) and CCS+CCM (χ2= 1.067, P = 0.302) had no significant effects on responses of C. suppressalis males. However, the males were predominantly attracted to the control than to Z11-18:OH (χ2= 32.267, P < 0.001), CM (χ2= 17.067, P < 0.001), CS+CM (χ2= 38.400, P < 0.001), and CCM (χ2= 13.067, P < 0.001) treatments. The results suggested that Z11-18:OH was the major component of C. medinalis to inhibit responses of C. suppressalis males.
Avoidance effects of seven lures on the responses of male C. suppressalis in an olfactometer (two choices). CCM means commercial C. medinalis sex pheromone; CCM+CCS means combination of commercial C. medinalis and C. suppressalis sex pheromones; CM means the four components of C. medinalis sex pheromone (Z13-18:Ald, Z13-18:OH, Z11-18:Ald, Z11-18:OH=100:36:11:24); CS+CM means combination of the components of C. suppressalis and C. medinalis sex pheromones (Z11-16:Ald, Z13-18:Ald, Z9-16:Ald, Z13-18:OH, Z11-18:Ald, Z11-18:OH=46:106:5:36:11:24). The concentrations of the lures are 1 μg/μl except the two commercial sex pheromones. The control (CK) is redistilled hexane. Significance levels of χ 2 (χ 2 test) indicated by *** (P < 0.001) or NS (no significant difference). n = 30.
Adding Z11-18:OH and Z13-18:OH to the CCS Significantly Decreased the Captures of C. suppressalis Males in the Field Experiment
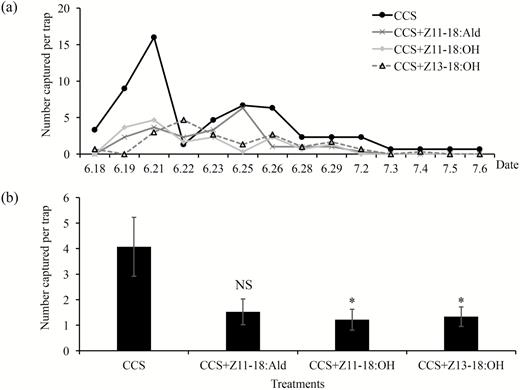
We examined the inhibition effects of the sex pheromone components of C. medinalis on the response of C. suppressalis males in the field. The results showed that adding Z11-18:OH (P < 0.05) or Z13-18:OH (P < 0.05) to the CCS inhibited the capture of C. suppressalis males in the field (Fig. 4). However, compared with control treatment (CCS), adding Z11-18:Ald (P = 0.058) had no significant inhibition effect on C. suppressalis males. This suggested that adding Z11-18:OH or Z13-18:OH to the CCS could significantly inhibit the attraction of CCS to C. suppressalis males in the field.
Dynamic linear graph (a) and summary graph (b) of number of C. suppressalis males captured by commercial C. suppressalis sex pheromone alone (CCS) and combined with the components of C. medinalis sex pheromone (Z11-18:Ald, Z11-18:OH, Z13-18:OH) in Jiangxi, China, from June to July 2014. The difference of capture of C. suppressalis males between treatments and control treatment were analyzed with Mann–Whitney U-test. *P < 0.05; NS, no significant difference.
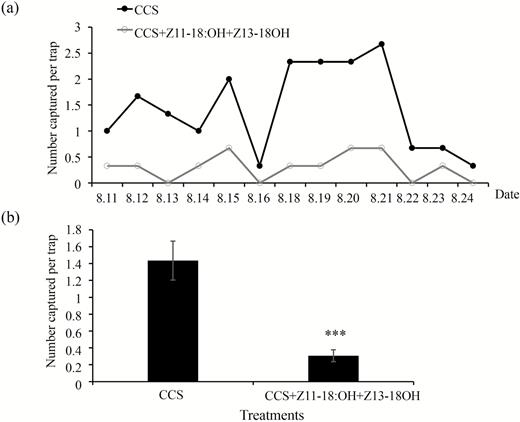
Furthermore, we investigated the response of C. suppressalis males to the combination of Z11-18:OH, Z13-18:OH, and CCS in the field experiment. We also found that adding both Z11-18:OH and Z13-18:OH (P < 0.001) to the CCS could significantly inhibit the attraction of CCS to C. suppressalis males in the field (Fig. 5). The means of total captures by CCS without and with both Z11-18:OH and Z13-18:OH were 18.66 and 2.33, respectively.
Dynamic linear graph (a) and summary graph (b) of number of C. suppressalis males captured by commercial C. suppressalis sex pheromone alone (CCS) and CCS plus individual components (Z11-18:OH and Z13-18:OH). The difference of capture of C. suppressalis males between treatments and control treatment were analyzed with Mann–Whitney U-test. ***P < 0.001.
Discussion
In this study, we found that mixing the sex pheromones of C. suppressalis and C. medinalis interfered with the capture of C. suppressalis males in the field experiments. Our finding was consistent with the result of Gemeno et al. (2006), which showed that mixing sex pheromones of two Lepidoptera species resulted in significantly lower captures of one of the species. Similarly, attraction of Tetanolita mynesalis (Walker) (Lepidoptera: Noctuidae) to its own pheromone was inhibited when mixed with the sex pheromone of Lacinipolia renigera (Stephens) (Lepidoptera: Noctuidae) (Haynes et al. 2002) and the sex pheromone of Adoxophyes orana (Fischer von Röslerstamm) (Lepidoptera: Corynidae) inhibited the attraction of Cydia pomonella (L.) (Lepidoptera: Tortricidae) (Potting et al. 1999). Such phenomena of inhibition or antagonism in the sex pheromone systems of several insect species probably contribute to the reproductive isolation of closely related species (same genus; Cardé et al. 1977, Borden 1997, Cardé and Haynes 2004). In addition, pheromone inhibition or antagonism may occur between species that are not closely related (different genera). For instance, Lopez et al. (1990) found that multispecies sex pheromone traps caused a reduction of baiting in more than one species compared to the baiting in individual species traps. Our results also demonstrated that CCM inhibited the attraction of C. suppressalis. Therefore, there is great risk of using two sex pheromones of insect pests together in controlling and monitoring these pests in the field.
Sex pheromones in many insect species are composed of multiple components. The permeation of an individual component in the air can often disrupt the sexual communication between male adult and female adult (Witzgall et al. 1999). Our olfactory experiments and field tests showed that adding Z11-18:OH and Z13-18:OH to the traps baited with CCS caused antagonism in the attraction of C. suppressalis males. The results are similar to the studies done on the yellow stem borer Scirpophaga incertulas (Walker) (Lepidoptera: Pyralidae), which showed that Z-11-hexadecenol from S. incertulas sex pheromone inhibited the captures of C. suppressalis males (Cork and Basu 1996). Some alcohols have been shown to be either synergists or inhibitors of many insect pheromones. Yu et al. (2014) reported that 1-undecanol acted as sex pheromone synergist to enhance the attraction of male Grapholita molesta (Busck) (Lepidoptera: Tortricidae) pheromone traps. A previous study reported that Z-11-hexadecenol inhibited the attraction of male Mamestra brassicae (Lepidoptera: Noctuidae) (Struble et al. 1980). Likewise, the attraction of C. pomonella was inhibited by Z-9-tetradecenol (Chisholm et al. 1983). Our results indicated that the alcohols Z11-18:OH and Z13-18:OH may act as inhibitors of the C. suppressalis sex pheromone.
We found that the combined usage of CCS and CCM in traps caused no capture of C. suppressalis males in field experiments. Our results also suggested that Z11-18:OH and Z13-18:OH could improve the efficiency of mating disruption by inhibiting the attraction of C. suppressalis males to CCS. In practice, ~6.64 million h m2 rice were damaged by herbivore arthropod pests in Jiangxi province in China, which scale was covered 66.84% for entirely damaged scale of crop lands during the years from 2010 to 2016 (Zhong et al. 2019). Currently, application of sex pheromone mating disruption techniques has become one of the most important and environment friendly methods to control lepidopteran pests on rice (Alfaro et al. 2009, Cui and Zhu 2016). Xu et al. (2019) tested the control effects of mating disruption on C. suppressalis and C. medinalis in Taihe county, Jiangxi province. The results showed that sex pheromone may efficaciously suppress the occurrence and damage of the two rice pests. In Yugan, Fengcheng, Shanggao, Jishui, and other counties, the sex pheromone management effects on C. suppressalis and C. medinalis were significant, reaching 60 and 40% damage reduction, respectively (Zhong et al. 2019). In order to reduce the labor cost and the number of monitoring traps, the combination of the two traps has been popularly used to capture pests colonizing in same niche (Zhou et al. 2012, Zhu et al. 2015). However, mixed usage of the different insect sex pheromones will bring the capture loss and even repellent effect on target insects, which would cause great hidden dangers to the outbreak of these target insects according to our results.
In summary, our research results are important to the guidance of agricultural production for the mixed use of sex pheromones of two major rice pests and play an increasingly important role in IPM. Thus, we do not suggest the usage of sex pheromones of these two species together in the same trap. In addition, we also suggest that Z11-18:OH and Z13-18:OH could be potential repellents or antagonists of C. suppressalis sex pheromone, which may contribute to biotechnological control with sex pheromone-mediated mating disruption in future.
Acknowledgments
The authors are grateful to Zhiwen Yao for assistance in arthropod sampling. We would like to thank DBMediting for professional English language editing services. We also thank Su Wang and Haosu Cong for comments on the manuscript. The financial support for this research was granted by the National Key R&D Program of China (Project No. 2017YFD0301604, 2016YFD0200808 and 2017YFD0200400). M.L. acknowledges the support from the China Scholarship Council (CSC) (File No. 201708360095).
Open Entomology
This paper is part of an Open Entomology collection in the Journal of Insect Science. Papers in the collection were submitted to a preprint server upon submission to the journal, have publicly available data when applicable, and have peer review information published as supplemental files, including the original version of the paper, decision letters and reviewer comments, and revised versions.
Data Availability
Data from this study are available at Harvard Dataverse: DOI: 10.7910/DVN/ASLDNA (Liang et al. 2020).