Abstract
Purpose
To determine the prevalence and sociodemographic and hospitalization history of genetic conditions in a sample of inpatients in a pediatric hospital in 2017, and to compare results with unpublished studies from 1985, 1995, and 2007.
Methods
Two weeks of admissions were classified according to a pre-existing categorization, based on genetic etiology, encompassing chromosomal and monogenic conditions, multifactorial (MF) conditions, and no known genetic cause.
Results
In 2017, 299 (16%) patients had chromosomal or monogenic conditions, 6–7% more than 2007 and 1995, but similar to 1985. Autosomal dominant (AD) conditions increased from <2% previously to 6% in 2017 (p < 0.001). MF conditions comprised the majority throughout, increasing from 45% to 54%. Age at admission was highest in autosomal recessive (AR) and X-linked categories in 1995, 2007, and 2017, reflected in their high number of previous admissions, while the AD, MF, and nongenetic categories were the youngest with similar lengths of stay and previous admissions.
Conclusion
Conditions with a genetic contribution account for over half of pediatric inpatients. Since 1985, there have been many changes in age at admission and length of stay, but it is the increasing prevalence of AR, AD, and MF conditions that is important when considering future service provision.
Similar content being viewed by others
INTRODUCTION
Advances in genomic medicine have led to an increase in the knowledge of genetic causes of disease1,2 and consequently, more conditions are being classified as having a genetic basis. The contribution of genetic factors to the etiology of morbidity and mortality in pediatric patients has been studied in a number of ways over many years.3,4,5,6,7,8 Most studies have utilized either hospital-based medical record reviews3,4,5,9,10,11,12 and/or analysis of administrative data coded with the International Classification of Diseases (ICD).6,7,13,14 These studies have explored the impact of genetic disease on various admission types, including pediatric inpatients, emergency departments,15,16 intensive care/high risk units,17,18,19,20,21 rehabilitation centers,22 and through state-wide population-based studies.
There are many different estimates of the genetic contribution to pediatric hospitalizations, derived from direct review of the medical records.3,4,5,9,10,11,12 A landmark study of pediatric hospitalizations published almost 40 years ago reported that 4.5% of all admissions were due to monogenic conditions, while 53% of all admissions had a genetic or partially genetic cause.4 Fifteen years ago, a hospital-based review of 5747 admissions found that 11% of admitted patients had either a single-gene or chromosomal condition and 70% had an underlying condition that was known to be at least partly genetically determined.5 Differences between classification systems, methods of categorization, definition of genetic conditions, populations and sources of data have contributed to the variation seen across these studies.
At the Royal Children’s Hospital (RCH), Melbourne, Australia, in order to review the contribution of genetics to hospital admissions, the same classification system has been utilized over four time periods, 1985, 1995, 2007, and 2017. The 2017 review of medical records was undertaken using electronic medical records, but otherwise, the methodology has been the same. As most of the published data are more than a decade old and conducted prior to the extensive use of next-generation sequencing and electronic health records, the true impact of pediatric genetic conditions may be underestimated.8 The aims of this study were to (1) determine the current prevalence of different categorizations of genetic and nongenetic conditions in a sample of patients admitted to RCH in 2017, (2) describe sociodemographic characteristics of inpatients with these conditions and their hospitalization history, and (3) compare all these results with data collected in the three earlier unpublished RCH studies.
MATERIALS AND METHODS
Study population
This descriptive study was undertaken at the RCH, Melbourne, in conjunction with the Murdoch Children’s Research Institute (MCRI). The RCH is the major specialist pediatric hospital in Victoria and is internationally recognized as a leading center for pediatric treatment, teaching, and research.23 The MCRI is the research institute located in the hospital and encompasses Victorian Clinical Genetics Services, the clinical genetics service for RCH and regional Victoria.
The electronic medical records of all admitted patients less than 18 years of age, who were discharged from the RCH between 1 and 14 March 2017, were reviewed. This period was selected to ensure consistency with the previous three reviews undertaken in 1985, 1995, and 2007. Admissions were excluded if they were overnight accommodation admissions, as patients with this admission type may have been accommodated before, during, or after their acute or same-day service, with no clinical services provided during these admission types.24 For instance, patients residing in a rural setting may be accommodated prior to their admission. In addition, admissions were excluded if the patient was transferred from the emergency observation ward (considered admitted episode) to an acute ward on the same day, for the same condition. Patients were identified through the RCH patient administrative database and provided by the health information service.
Generalizability of the two-week sample
The total number of admissions for the year, for 20 different conditions, was obtained in both 2007 and 2017. The proportion of admissions for each of these conditions was compared with the proportion in the two-week sample period. There were no differences found at all, confirming that this period in March was representative of the whole year.
Data classification
Eligible admissions were assigned to one of six categories based on the pre-existing classification of conditions developed by an expert panel of clinical geneticists, consistently used in the 1985, 1995, and 2007 studies (Table 1). Category 1 comprised chromosomal conditions and included all cytogenetically visible chromosome abnormalities and copy-number variants that encompassed multiple genes. Mendelian conditions were classified into categories 2–4, with category 2 comprising autosomal recessive (AR) conditions, category 3 X-linked conditions, and category 4 autosomal dominant (AD) conditions. Category 5 comprised multifactorial conditions, and included all conditions for which there is known to be a genetic contribution in at least a proportion of cases, including polygenic conditions (e.g., asthma) as well as conditions where a subset of cases are caused by a single high penetrance gene variant (e.g., retinoblastoma). Note that cases where file review showed clear evidence of Mendelian inheritance (e.g., bilateral retinoblastoma with family history) were classified as AD rather than multifactorial. Category 6 (nongenetic conditions) comprised conditions where a combination of clinical diagnosis and file review did not reveal evidence of a genetic contribution, e.g., trauma and infections.
To assist with the classification of new conditions not already in the pre-existing list, information was obtained from two databases: OMIM25 and Pictures of Standard Syndromes and Unidentified Malformations (POSSUM).26 For cases that were difficult to assign to a category, consultation was sought from a clinical geneticist and/or epidemiologist (who is an expert in genetics and congenital anomalies). To ensure the validity of the data, regular meetings were held with the research team to verify the list of categories and corresponding diseases prior to analysis.
The classification system was hierarchical, such that a patient with both cystic fibrosis (AR) and autism (multifactorial) was assigned to category two (AR). Admissions containing more than one condition in the same category were assigned to the condition listed as the principal diagnosis on the discharge summary or the first listed diagnosis. If a genetic condition was found in the medical record review but was not a reason for admission, then it was recorded that the admission was unrelated to that condition. For example, a patient with β-thalassemia (AR), admitted for treatment of an arm fracture due to a motor vehicle accident (nongenetic illness), would be classified in the AR category, but the admission would be recorded as unrelated to the genetic condition. This allowed the researcher to identify how many patients were admitted for treatment of their condition or for unrelated health problems.
Data collection and analysis
The electronic medical record (EMR) Epic was reviewed for diagnoses, comorbidities, and family history for each patient. All diagnoses, even those not directly related to the episode of care, were included. However, comorbidities had to be current (still existing or being treated at the time of the admission) to be eligible for inclusion. The review of the EMR included looking through the discharge summary and progress notes for the corresponding admission. The record was also reviewed for evidence of genetic counseling and/or clinical genetic consultation for a diagnosis to assist with the classification of the inheritance of genetic conditions. Any tests that were ordered within the admission were checked to determine if they confirmed the presence or absence of a specific condition. As some genetic tests may have a turnaround time of several weeks to months,8,27,28 admissions occurring after the specified timeframe were reviewed to assist with the classification of suspected conditions that might have been diagnosed after the study. However, for a condition to be included in the study, the signs and symptoms had to be present and documented in the March 2017 sample. For example, a patient with signs and symptoms of asthma in March 2017 (documented as suspected, but not conclusive), which was confirmed at a later date (June 2017), was included because asthma may require more than one episode of respiratory symptoms for a clinician to make a definitive diagnosis.
Data pertaining to patient demographics were extracted from the patient administrative database by the health information service at RCH.
Statistical analysis was performed using STATA (version 14), Epi Info (version 7), and Microsoft Excel, using descriptive statistics, chi-square analyses, and a Student’s t-test to compare variables between the six categories. Statistical comparisons were possible with the 2007 data due to the similarity of the study methodologies.
The RCH Human Research Ethics Committee (HREC) and La Trobe University Human Research Ethics Committee provided ethics committee approval for this research (HREC reference 38004A).
RESULTS
Across the four 2-week periods, there were a total of 6592 admitted episodes for which the reason for admission was classified into 648 different conditions within the six etiological categories (Supplementary Table 1). In summary, there were 39 chromosomal, 59 AR, 28 X-linked, 74 AD, 225 multifactorial, and 223 nongenetic conditions.
For the 2-week period in 2017 there were 1908 admitted episodes. Twenty-six were excluded as they were duplicates or overnight accommodation admissions, leaving 1882 to be classified (Table 2). These admissions represented 1715 patients, some having more than one admission within the sample period.
In total, there were 299 (15.9%) admissions for single-gene and chromosomal conditions (categories 1–4) and 1009 (53.3%) for multifactorial conditions. (Table 2). The other 574 (30.5%) were admissions for conditions with no known genetic contribution.
Although comparable 2-week periods in March were examined, the overall number of admissions in 2017 was higher than in 2007. The largest changes over the entire study period were the decrease in AR from 9.4% in 1985 to 4.8% in 2017 (p < 0.001), increase in AD from 1.3% to almost 6% and decreasing trends across the nongenetic category (Table 2). Overall, simple genetic conditions (categories 1–4) increased by 6–7% from 1995 and 2007 (p < 0.001). There was no marked change in the proportion of multifactorial conditions between the years.
Diagnoses in genetic categories
There was a total of 443 different conditions diagnosed among the 1882 admissions during the two-week period at the RCH in 2017, of which 213 were classified as genetic. Of the 56 admissions of patients with chromosome conditions, 28.8% (n = 15) had trisomy 21 and 37.5% (n = 21) had chromosome deletions (e.g., 3q21.1q23 deletion syndrome, 3p deletion syndrome, and 17p13.2 deletion). The remaining 20 admissions with chromosome conditions included Turner syndrome, Klinefelter syndrome, and various duplications. The most common AR condition was cystic fibrosis, accounting for 15.4% (n = 14 of 91) of admissions in this category. Hunter syndrome was the most frequent admission in the sex-linked category (35%, n = 14 of 40) and neurofibromatosis type 1 in the AD category (10.8%, n = 12 of 111). Of the conditions in the multifactorial category, autism spectrum condition, hypospadias, and cancers were the most frequently occurring admissions.
Admissions directly related to genetic category
Eighty-five percent (n = 1607) of admissions were directly related to the category assigned. That is, the condition or its complications was directly treated during the episode of care. Patients with chromosomal and AD conditions had the highest proportion of unrelated admissions to the category assigned.
Characteristics of patients
Gender and age
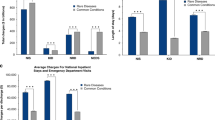
Overall, in the 2017 sample, there were more admissions of male children (56.2%) compared with females (43.8%) (Table 3). More than half of all admissions were for males in all categories except for chromosomal and AD categories. These data were consistent over the four sample time periods, with males more common across all six categories (Fig. 1a).
(a) Gender by category (1985, 1995, 2007, 2017). (b) Mean age by category (1985, 1995, 2007, 2017). (c) Mean length of stay by category (1985, 1995, 2007, 2017). (d). Mean previous admissions by category (1985, 1995, 2007, 2017). Previous admission denominator is the number of patients in the sample (e.g., for 2017, n = 1715). AD autosomal dominant, AR autosomal recessive, Chr chromosomal, MF multifactorial, NG nongenetic, XL sex linked.
One-third of patients in the 2017 sample (n = 624) were aged between 1 and 4 years (Table 3); yet admissions in the genetic categories 1–4 were more likely to involve older children, with 60.5% (n = 181 of 299) aged greater than five years. In contrast, 59.1% (n = 339 of 574) of patients with nongenetic related admissions were aged between zero and four years. The average age for genetic categories 1–4 was 7.9 years (SD 5.6), compared with 5.4 years (SD 5.1) for nongenetic categories (p < 0.001). Compared with 1985, the mean age of admissions in all categories was markedly higher across the other three time periods. The age at admission for AR and X-linked conditions increased the most. (Fig. 1b). The lowest mean age was for patients in the nongenetic category, this having decreased significantly from 2007 to 2017 (p < 0.01).
Socio-Economic Indexes for Areas (SEIFA)
There is little disparity between the socioeconomic position of patients with genetic and nongenetic conditions, with patients in both groups more likely to be ranked in the top two quartiles (Table 3). There were no statistically significant differences between genetic and nongenetic admissions observed between 2007 and 2017. These data were not available for the earlier studies.
Hospitalization
Length of stay
The average length of stay (ALoS) in 2017 was found to be highest among category 2, AR (3.9 days), followed by category 4, AD (3.6 days) (Table 3). The ALoS across all categories was no more than 3.9 days but huge variation occurred as noted by the large SDs. Since the 1985 study sample, the ALoS decreased in most categories over time (Fig. 1c).
In 2017, 55% (n = 1036) of admissions were same-day presentations and 45% (n = 846) overnight admissions with a minimum LoS of at least one night. Patients with multifactorial conditions were significantly more likely to have a same-day admission than nongenetic patients (p < 0.001). Admissions with X-linked (67.5%) and AR conditions (65.2%) had the most same-day presentations. In contrast, patients with simple genetic and multifactorial conditions had a greater proportion of admissions with a LoS of more than 7 days, while the nongenetic category had a higher proportion of admissions with a LoS between 1 and 6 days.
Previous admissions
In 2017, patients with an AR or a sex-linked condition had a higher average number of previous admissions (>15) than all other categories (Table 3). This is consistent with 1985, 1995, and 2007 findings (Fig. 1d). The average number of previous of admissions for genetic categories 1–4 has increased since 2007; however, no significant changes were noted.
DISCUSSION
This study provides the first description of the current prevalence of different categories of genetic and nongenetic conditions in a large metropolitan children’s hospital using an electronic medical record review. The 2017 data show that 16% of admissions in a two-week sample of patients aged 0–18 years had a chromosomal or Mendelian genetic condition and 53.6% had a multifactorial condition with a partially genetic cause. This is lower than results previously reported by McCandless et al.,5 but consistent with the findings from Hall et al.4 despite his study being undertaken four decades ago.
Compared with 2007, there were increases in all genetic categories, particularly in the number of chromosomal (category 1) and AD (category 4) conditions. The observed increase seen in these categories may result from increasing maternal age and technological advances in genetic screening and genomic testing.29 The number of AD conditions is markedly higher in 2017 with many being single cases (n = 38) of rare conditions arising from de novo gene variants, which may well have been detected using advanced genomic technologies that became available in the preceding decade.30 Unfortunately, it was not possible to determine how the diagnoses were made across the years, but it is well recognized that there has been an increasing frequency of both the discovery and diagnoses of rare Mendelian conditions since the introduction of ES and GS.31 Since 2013, data from the National Institutes of Health (NIH) Centers for Mendelian Genomics show that these genomic technologies have discovered almost three times the number of genes as previously. In line with this, our data show three times as many diagnosed AD conditions admitted in 2017 than previously.
In 1985, the number of AR conditions at the RCH was much higher than in more recent years, specifically due to the large number of admissions for thalassemia (n = 114) and cystic fibrosis (n = 59). Relocation of the Victorian thalassemia service to another pediatric service, and improved management of cystic fibrosis, including the introduction of newborn screening, undoubtedly account for the decrease in the AR admissions.
In regard to chromosomal conditions, the introduction of the use of chromosomal microarrays (CMA) has increased the detection of submicroscopic chromosomal anomalies (DNA copy-number variants).31,32 CMA was introduced as a first tier test in the pediatric setting in 2006 for intellectual disability or multiple anomalies32,33,34 and this may contribute to the large proportion of deletions (37.5%) in the chromosomal category of this 2017 study.
Over half of the admissions in the 2017 sample were due to multifactorial conditions, with no change since 2007; however, a 10% increase is seen within this category between 1985 and 1995. Some of this is due to same-day admissions for allergy and food challenges, not seen in the earlier years. Another contributing factor is the increase in admissions, again many being same day, for chemotherapy treatment, from 4.3% in 1985 to 10.8% in 2017. According to the Australian Institute of Health and Welfare, in 2016–2017 the majority of same-day acute separations in public hospitals were for chemotherapy.35 This, coupled with changing practices in medical treatment, is likely to be increasing the numbers observed within the study.
Another explanation for the decrease seen in the nongenetic illness category is that the last four decades has seen a shift from admissions being due to infections and trauma, to more chronic debilitating diseases, most of which have a genetic or partially genetic cause, For instance, in 1985 infections such as tonsillitis and gastroenteritis accounted for 23.2% of admissions. In 2017 infections only accounted for 7.2% of admissions. The inclusion of more untreated comorbidities such as asthma, allergies, and autism is also an influential factor.
Inpatient and hospitalization characteristics
This study also examined the inpatient and hospital characteristics of children with genetic conditions. Consistent with previous research, males were more likely to have a genetic condition, compared with females. Children with single-gene and chromosomal conditions were found to have a higher mean age (7.9 years), compared with patients with multifactorial (7.2 years) and nongenetic conditions (5.4 years). This is similar to the findings of McCandless et al. where the mean age was 6.1 years for single-gene conditions, and 5.4 years for those patients with nongenetic conditions.5 Compared with the three previous studies, there is an increasing mean age observed, explained by medical advances that are likely to improve the life expectancy of children living with these monogenic conditions.20 As a result, patients with genetic conditions are living longer, increasing use of hospital resources compared with nongenetic patients.
Length of stay
As stated above, there were increasing numbers of patients with same-day admissions. This is reflected in the reduced ALoS in category 5 (multifactorial), which has decreased since 1985 and 1995 from 11.4 and 4.8 days respectively to 2.9 days in 2017. In this category, the majority of patients were admitted for same-day chemotherapy, while patients with inflammatory bowel disease attended for same-day infliximab transfusions and others for food and drug challenges. This downward trend has previously been reported, with factors such as changes in admission patterns, clinical practice, financial pressures, and advances in medical technology thought to contribute to this decline.36 In Australia, the ALoS for all age groups has decreased since 2004–2005 from 3.4 days to 2.8 days in 2016–2017,37 while the rate of same-day hospitalizations continues to increase.38 This is comparable with the findings reported in our study. This trend was also apparent in the population study by Dye et al., where it was reported that in 2006, 41% of admissions for patients with single-gene and chromosomal conditions were same-day admissions.6 Regardless of the increasing trend of same-day admissions, patients with single-gene and chromosomal conditions require longer hospitalizations compared with other patients. This supports the findings of earlier studies that found genetic patients had admissions twice as long as nongenetic patients.6,7
Previous admissions
In 2017, there was an increase in the mean number of previous admissions for genetic categories 1–4, while the mean number in the nongenetic category has remained consistently very low over the four sample periods. The average admission number for single-gene and chromosomal conditions in 2017 was 26.7 (SD = 69.2), the large SD observed being due to outliers. This number is an increase in what has been previously reported by Hall et al., at 5.3 admissions,4 and 2.6 admissions in the study by Dye et al.6 However, the low mean number of previous admissions in the study by Dye et al. is due to the study only including previous admissions occurring between 2000 and 2006,6 while this study and that of Hall et al. counted all previous admissions in the patient’s lifetime.4 The increase in number of previous admissions observed in 2017 is consistent with the chronic and relapsing course of most genetic conditions.10 This is not surprising given the changes to hospital practice and improvements to treatment, resulting in people with genetic conditions living longer.
Strengths and limitations
The strengths of this current study include the large number of admissions examined carefully, the rigor of the categorization, and the ability to review the complete medical record for an underlying genetic condition, even when not directly treated in the episode of care. In addition, the ability to use the Epic EMR enabled 100% of the eligible admissions to be reviewed, with no missing records. Also, the functionality of the EMR ensured there was a consistent approach for abstracting information from the medical record for each admission.
The existence of equivalent data from 2-week periods in 1985, 1995, and 2007 provides a unique opportunity to observe trends in disease and hospital patterns, but this strength is also a weakness, because equivalent patients may have been classified differently in different decades. For example, a patient in 2017 admitted with X-linked epileptic encephalopathy due to PCDH19 (X-linked) would likely have been classified as epilepsy (multifactorial) in previous decades. It is also acknowledged that the distinction between multifactorial disorders and nongenetic disorders is not always clear, and that even conditions such as infection and trauma may have contributing genetic factors.
Another minor limitation of this study is that it included only inpatients from one pediatric hospital and excluded patients seen in the emergency and outpatient setting. Nevertheless, the RCH is the major pediatric hospital, including admissions from all over the state, enhancing the generalizability of the results to a wider pediatric population.
It is possible that differences between the characteristics and experiences of the researchers undertaking the categorization of conditions may have impacted the results. For example, the two earliest studies were undertaken by medical students, while the current and 2007 study were conducted by health information management students who have expertise in medical record abstraction and extensive knowledge of the ICD-10-AM classification system. Potential disparities in classification were addressed by involving a clinical geneticist and epidemiologist expert in congenital anomalies, who assisted with the categorization and verification of disease throughout the classification process in these two study periods. It was more difficult to compare the results between the 1985 and 1995 cohorts, and therefore most comparisons were made between the 2007 and 2017 studies when the procedures and characteristics of the researchers were similar.
Last, categorization was based exclusively on the documentation within the medical records. As the current study utilized an EMR review, potential biases may include notes being copied and pasted when they do not relate to particular episodes of care, not end dating diagnoses that appear on automated patient history sections, and failing to update allergies and family history.39 This becomes problematic when deciphering what diagnoses may be current and those that are no longer being actively treated. As a result, the use of an EMR may overestimate the true impact of “current” conditions. Further, the experience of the clinician may have an impact on the quality of information within the record. For instance, clinicians with increased knowledge in genetics and genetic causes of disease may be more likely to refer patients for further testing. This may increase the number of incidental findings of genetic conditions.
Conclusions
This study has demonstrated that over half of the RCH admissions in the study period in 2017 had an underlying genetic contribution, with the majority of these admissions directly related to the episode of care. Since 2007, the percentage of patients with single-gene and chromosomal conditions has significantly increased, from 10% to almost 16% in 2017. Patients with genetic conditions had more admissions and stayed longer in hospital than those patients with nongenetic conditions. An increasing mean age at admission since 1985 was observed for patients with monogenic conditions, indicating that patients are living longer. With genetic technological advances, more discoveries are being made on the genetic causes of disease, leading to an increased detection of genetic conditions caused by nontraditional inheritance such as de novo pathogenic variations, copy-number variants, nucleotide repeats, and epigenetic, imprinting, and mitochondrial conditions.40 Future research should assess the magnitude of these specific genetic etiologies, updating the current categorization system to reflect nontraditional genetic causes of disease. Ultimately, the data generated from this study provide an up to date estimate of the current prevalence of genetic conditions in the pediatric population, confirming that these are common in the pediatric inpatient setting.
References
Mattick JS, Dziadek MA, Terrill BN, et al. The impact of genomics on the future of medicine and health. Med J Aust. 2014;201:17–20.
Stranneheim H, Wedell A. Exome and genome sequencing: a revolution for the discovery and diagnosis of monogenic disorders. J Intern Med. 2016;279:3–15.
Day N, Holmes LB. The incidence of genetic disease in a university hospital population. Am J Hum Genet. 1973;25:237–246.
Hall JG, Powers EK, McLlvaine RT, et al. The frequency and financial burden of genetic disease in a pediatric hospital. Am J Med Genet. 1978;1:417–436.
McCandless SE, Brunger JW, Cassidy SB. The burden of genetic disease on inpatient care in a children’s hospital. Am J Hum Genet. 2004;74:121–127.
Dye DE, Brameld KJ, Maxwell S, et al. The impact of single gene and chromosomal disorders on hospital admissions of children and adolescents: a population-based study. Public Health Genomics. 2011;14:153–161.
Yoon PW, Olney RS, Khoury MJ, et al. Contribution of birth defects and genetic diseases to pediatric hospitalizations. A population-based study. Arch Pediatr Adolesc Med. 1997;151:1096–1103.
Wojcik MH, Schwartz TS, Yamin I, et al. Genetic disorders and mortality in infancy and early childhood: delayed diagnoses and missed opportunities. Genet Med. 2018;20:1396–1404.
Scriver CR, Neal JL, Saginur R, et al. The frequency of genetic disease and congenital malformation among patients in a pediatric hospital. Can Med Assoc J. 1973;108:1111–1115.
Carnevale A, Hernandez M, Reyes R, et al. The frequency and economic burden of genetic disease in a pediatric hospital in Mexico City. Am J Med Genet. 1985;20:665–675.
Penchaszadeh VB. Frequency and characteristics of birth defects admissions to a pediatric hospital in Venezuela. Am J Med Genet. 1979;3:359–369.
Lialiaris T, Mantadakis E, Kareli D, et al. Frequency of genetic diseases and health coverage of children requiring admission in a general pediatric clinic of northern Greece. Ital J Pediatr. 2010;36:9.
Baird PA, Anderson TW, Newcombe HB, et al. Genetic disorders in children and young adults: a population study. Am J Hum Genet. 1988;42:677–693.
Soltan HC, Craven JL. The extent of genetic disease in hospital populations. Can Med Assoc J. 1981;124:427–429.
Kumar P, Radhakrishnan J, Chowdhary MA, et al. Prevalence and patterns of presentation of genetic disorders in a pediatric emergency department. Mayo Clin Proc. 2001;76:777–783.
Meguid N, El-Bayoumi S, Hamdi N, et al. Prevalence of genetic disorders in pediatric emergency department Al Galaa teaching hospital. Pakistan J Biol Sci. 2003;6:1961–1965.
Cunniff C, Carmack JL, Kirby RS, et al. Contribution of heritable disorders to mortality in the pediatric intensive care unit. Pediatrics. 1995;95:678–681.
FitzPatrick DR, Skeoch CH, Tolmie JL. Genetic aspects of admissions to a paediatric intensive care unit. Arch Dis Child. 1991;66:639–641.
Hudome SM, Kirby RS, Senner JW, et al. Contribution of genetic disorders to neonatal mortality in a regional intensive care setting. Am J Perinatol. 1994;11:100–103.
Soneda A, Teruya H, Furuya N, et al. Proportion of malformations and genetic disorders among cases encountered at a high-care unit in a children’s hospital. Eur J Pediatr. 2012;171:301–305.
Stevenson DA, Carey JC. Contribution of malformations and genetic disorders to mortality in a children’s hospital. Am J Med Genet A. 2004;126A:393–397.
O’Malley M, Hutcheon RG. Genetic disorders and congenital malformations in pediatric long-term care. J Am Med Dir Assoc. 2007;8:332–334.
Royal Children’s Hospital. 2018. https://www.rch.org.au/rch/about/. Accessed 25 Aug, 2018.
Australian Institute of Health and Welfare. Metadata Online Registry. 2018. http://meteor.aihw.gov.au/content/index.phtml/itemId/374149. Accessed 12 Sep, 2018.
OMIM. Baltimore, MD: Johns Hopkins University Center for Medical Genetics, 1996. https://www.ncbi.nlm.nih.gov/omim (2018).
POSSUM. Melbourne: Victorian Clinical Genetics Service and Murdoch Childrens Research Institute, 1984. https://www.possum.net.au/ (2018).
Shashi V, McConkie-Rosell A, Rosell B, et al. The utility of the traditional medical genetics diagnostic evaluation in the context of next-generation sequencing for undiagnosed genetic disorders. Genet Med. 2014;16:176–182.
Malam F, Hartley T, Gillespie MK, et al. Benchmarking outcomes in the neonatal intensive care unit: cytogenetic and molecular diagnostic rates in a retrospective cohort. Am J Med Genet A. 2017;173:1839–1847.
Consultative Council on Obstetric and Paediatric Mortality and Morbidity. Congenital anomalies in Victoria 2015–2016. Melbourne, VIC, Australia: Consultative Council on Obstetric and Paediatric Mortality and Morbidity; 2018.
Boycott KM, Vanstone MR, Bulman DE, et al. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14:681–691.
Chong JX, Buckingham KJ, Jhangiani SN, et al. The genetic basis of mendelian phenotypes: discoveries, challenges, and opportunities. Am J Hum Genet. 2015;97:199–215.
Bruno DL, Ganesamoorthy D, Schoumans J, et al. Detection of cryptic pathogenic copy number variations and constitutional loss of heterozygosity using high resolution SNP microarray analysis in 117 patients referred for cytogenetic analysis and impact on clinical practice. J Med Genet. 2009;46:123–131.
Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–64.
Bruno DL, Stark Z, Amor DJ, et al. Extending the scope of diagnostic chromosome analysis: detection of single gene defects using high-resolution SNP microarrays. Hum Mutat. 2011;32:1500–1506.
Australian Institute of Health and Welfare. Admitted patient care 2017-2018: Australian hospital statistics. Canberra, ACT, Australia: Australian Institute of Health and Welfare; 2019.
Kalra AD, Fisher RS, Axelrod P. Decreased length of stay and cumulative hospitalized days despite increased patient admissions and readmissions in an area of urban poverty. J Gen Intern Med. 2010;25:930–935.
Australian Institute of Health and Welfare. Admitted patient care 2015-2016: Australian hospital statistics. Canberra, ACT, Australia: Australian Institute of Health and Welfare; 2017.
Australian Institute of Health and Welfare. Australia’s Health 2016. Canberra, ACT, Australia: Australian Institute of Health and Welfare; 2016.
Casey JA, Schwartz BS, Stewart WF, et al. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. 2016;37:61–81.
Feldkamp ML, Carey JC, Byrne JLB, et al. Etiology and clinical presentation of birth defects: population based study. BMJ. 2017;357:j2249.
Acknowledgements
We gratefully acknowledge the following individuals for their cooperation and support: Sharon Lewis, Cathie Rose, Kerin Robinson, Catriona (Stanley) Gardiner, Allison Lusher, Karen Barry, and Kathy Kassin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gjorgioski, S., Halliday, J., Riley, M. et al. Genetics and pediatric hospital admissions, 1985 to 2017. Genet Med 22, 1777–1785 (2020). https://doi.org/10.1038/s41436-020-0871-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0871-9
Keywords
This article is cited by
-
Genomic newborn screening for rare diseases
Nature Reviews Genetics (2023)
-
Elusive variants in autosomal recessive disease: how can we improve timely diagnosis?
European Journal of Human Genetics (2023)
-
Preferences and values for rapid genomic testing in critically ill infants and children: a discrete choice experiment
European Journal of Human Genetics (2021)