Abstract
Objective
To assess whether different Gd-EOB-DTPA injection rates could influence the development of artifacts during the arterial phase of liver MRI studies.
Materials and methods
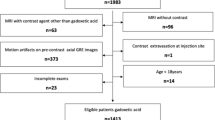
All Gd-EOB-DTPA liver MRI studies performed for different clinical indications at a single tertiary referral center were retrospectively evaluated. Each examination was acquired on a 1.5 T scanner with T1 In- and Out-of-Phase, T2 with and without fat-saturation, DWI, and 3D-T1 fat-sat dynamic sequences. Patients were divided into two groups according to the injection rate (1 ml/s and 1.5 ml/s). A single radiologist recorded the presence or absence of artifacts during different acquisition phases, respectively: (1) all examination; (2) only during the arterial phase; (3) only during the portal-venous phase; (4) both in arterial and portal-venous phases. From a total of 748 MRI studies performed, 229 were excluded due to the presence of artifacts during the entire examination. The remaining 519 MRI studies were divided into two groups according to the injection rate.
Results
The first group (flow rate = 1 ml/s) was composed by 312 (60.1%) patients and the second group (flow rate = 1.5 ml/s) by 207 (39.9%) patients. In the first group, 2 (0.6%) patients showed artifacts in all dynamic sequences; 13 (4%) only in the arterial phase, 16 (5%) only in the portal-venous phase, and 38 (12%) both in arterial and portal-venous phases; a total of 243 (78%) showed no artifacts. In the second group, 3 (1.5%) patients had artifacts in all dynamic sequences, 82 (40%) only in the arterial phase, 20 (10%) only in the portal-venous phase, and 53 (25%) both in arterial and portal-venous phases; a total of 49 (23.5%) showed no artifacts. A significant difference between the two groups regarding the absence of artifacts in all examination and the presence of artifacts only during the arterial phase was found (p < 0.001).
Conclusion
The development of artifacts during the arterial phase of Gd-EOB-DTPA liver MRI studies could be related to the injection rate and its reduction may help to decrease the incidence of artifacts.




Similar content being viewed by others
Abbreviations
- CM:
-
Contrast media
- DWI:
-
Diffusion-weighted imaging
- EMA:
-
European Medicines Agency
- ESGAR:
-
European Society of Gastrointestinal and Abdominal Radiology
- Gd-BOPTA:
-
Gadobenate dimeglumine
- Gd-EOB-DTPA:
-
Gadolinium ethoxybenzyl-diethylenetriaminepentaacetic acid
- MRI:
-
Magnetic resonance imaging
References
Semelka RC, Shoenut P, Kroeker MA et al (1992) Focal liver disease: comparison of dynamic contrast enhanced CT and T2-weighted fat-suppressed, FLASH and dynamic gadolinium-enhanced MR imaging at 1.5 T. Radiology 184:687
Semelka RC, Martin DR, Balci C et al (2001) Focal liver lesions: comparison of dual phase CT and multisequence multiplanar MR imaging including dynamic gadolinium enhancement. J Magn Reson Imaging 13:397–401
Bellin MF, Vasile M, Morel-Precetti S (2003) Currently used non-specific extracellular MR contrast media. Eur Radiol 13:2688–2698
Balci NC, Semelka RC (2005) Contrast agents for MR imaging of the liver. Radiol Clin N Am 43:887–898
van Montfoort JE, Stieger B, Meijer DK, Weinmann HJ, Meier PJ, Fattinger KE (1999) Hepatic uptake of the magnetic resonance imaging contrast agent gadoxetate by the organic anion transporting polypeptide Oatp1. J Pharmacol Exp Ther 290:153–157
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV (2009) Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 29:1725–1748
Semelka RC, Helmberger TK (2001) Contrast agents for MR imaging of the liver. Radiology 218:27–33
Neri E, Bali MA, Ba-Ssalamah A, Boraschi P, Brancatelli G, Alves FC, Grazioli L, Helmberger T, Lee JM, Manfredi R, Martì-Bonmatì L, Matos C, Merkle EM, Op DeBeeck B, Schima W, Skehan S, Vilgrain V, Zech C, Bartolozzi C (2016) ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol 26(4):921–931. https://doi.org/10.1007/s00330-015-3900-3(Epub 2015 Jul 21, PMID: 26194455, PMCID: PMC4778143)
Gupta RT, Iseman CM, Leyendecker JR, Shyknevsky I, Merkle EM, Taouli B (2012) Diagnosis of focal nodular hyperplasia with MRI: multicenter retrospective study comparing gadobenate dimeglumine to gadoxetate disodium. AJR Am J Roentgenol 199:35–43
Karam AR, Shankar S, Surapaneni P, Kim YH, Hussain S (2010) Focal nodular hyperplasia: central scar enhancement pattern using gadoxetate disodium. J Magn Reson Imaging 32:341–344
Park Y, Kim SH, Kim SH et al (2010) Gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI versus gadobenate dimeglumine (Gd-BOPTA)-enhanced MRI for preoperatively detecting hepatocellular carcinoma: an initial experience. Korean J Radiol 11:433–440
Ringe KI, Husarik DB, Sirlin CB, Merkle EM (2010) Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 195(1):13–28. https://doi.org/10.2214/AJR.10.4392(Preview PubMed PMID: 20566794)
Czervionke LF, Czervionke JM, Daniels DL, Haughton VM (1988) Characteristic features of MR truncation artifacts. AJR Am J Roentgenol 151(6):1219–1228 (PubMed PMID: 3263776)
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging JMRI 32(2):334–340
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271(2):426–434
Yoo JL, Lee CH, Park YS et al (2016) The short breath-hold technique, controlled aliasing in parallel imaging results in higher acceleration, can be the first step to overcoming a degraded hepatic arterial phase in liver magnetic resonance imaging: a prospective randomized control study. Investig Radiol 51(7):440–446
Kim YK, Lin WC, Sung K et al (2017) Reducing artifacts during arterial phase of gadoxetate disodium-enhanced MR imaging: dilution method versus reduced injection rate. Radiology 283(2):429–437
McClellan TR, Motosugi U, Middleton MS et al (2017) Intravenous gadoxetate disodium administration reduces breath-holding capacity in the hepatic arterial phase: a multi-center randomized placebo-controlled trial. Radiology 282(2):361–368
Rohrer M, Bauer H, Mintorovitch J et al (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig Radiol 40:715–724
Ringe KI et al (2010) Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 195(1):13–28. https://doi.org/10.2214/AJR.10.4392
Grazioli L et al (2018) Evaluation of incidence of acute transient dyspnea and related artifacts after administration of gadoxetate disodium: a prospective observational study. La Radiol Med 123(12):910–917. https://doi.org/10.1007/s11547-018-0927-y
Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK (2016) Triple arterial phase MR imaging with gadoxetic acid using a combination of contrast enhanced time robust angiography, keyhole, and viewsharing techniques and two-dimensional parallel imaging in comparison with conventional single arterial phase. Korean J Radiol 17(4):522–532. https://doi.org/10.3348/kjr.2016.17.4.522
Zech CJ, Vos B, Nordell A et al (2009) Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Investig Radiol 44:305–310
Motosugi U, Ichikawa T, Sou H et al (2009) Dilution method of gadolinium ethoxybenzyl diethylene-triaminepenta-acetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI). J Magn Reson Imaging 30:849–854
Tamada T, Ito K, Yoshida K, Kanki A, Higaki A, Tanimoto D, Higashi H (2011) Comparison of three different injection methods for arterial phase of Gd-EOB-DTPA enhanced MR imaging of the liver. Eur J Radiol 80(3):e284–e288. https://doi.org/10.1016/j.ejrad.2010.12.082(Epub 2011 Feb 5 PubMed PMID: 21296514)
McVeigh ER et al (1985) Noise and filtration in magnetic resonance imaging. Med Phys 12(5):586–591. https://doi.org/10.1118/1.595679
Huang SY et al (2015) Body MR imaging: artifacts, k-space, and solutions. Radiographics 35(5):1439–1460. https://doi.org/10.1148/rg.2015140289
Vikhoff-Baaz B et al (2001) Effects of k-space filtering and image interpolation on image fidelity in 1H MRSI. Magn Reson Imaging 19(9):1227–1234. https://doi.org/10.1016/s0730-725x(01)00456-8
Block KT et al (2008) Suppression of MRI truncation artifacts using total variation constrained data extrapolation. Int J Biomed Imaging. https://doi.org/10.1155/2008/184123
Bellin M-F (2006) MR contrast agents, the old and the new. Eur J Radiol 60(3):314–323. https://doi.org/10.1016/j.ejrad.2006.06.021
Funding
No supporting funding were employed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ippolito, D., Maino, C., Pecorelli, A. et al. Influence of injection rate in determining the development of artifacts during the acquisition of dynamic arterial phase in Gd-EOB-DTPA MRI studies. Magn Reson Mater Phy 34, 133–140 (2021). https://doi.org/10.1007/s10334-020-00857-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-020-00857-1