Article contents
Fabrication, mechanical property and in vitro bioactivity of hierarchical macro-/micro-/nano-porous titanium and titanium molybdenum alloys
Published online by Cambridge University Press: 18 June 2020
Abstract
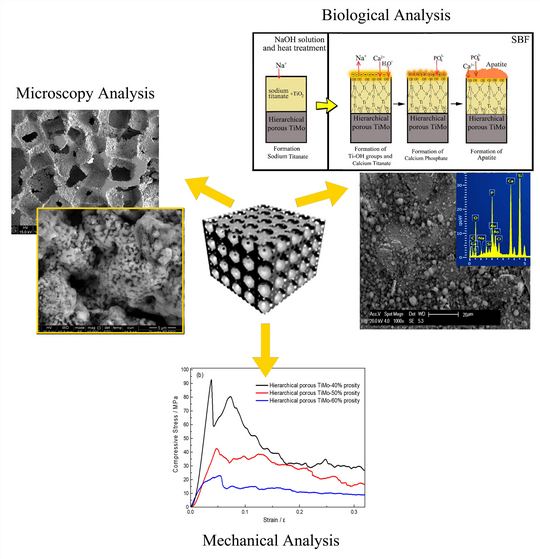
Novel three-dimensional (3D) hierarchical macro- to nano-porous titanium (Ti) and TiMo alloys with sufficient compressive strength (CS) were prepared using NaCl spacer and dealloying methods. The dealloying process was implemented by the heat treatment of TiCu and TiMoCu master alloys in Mg powders. The 3D-hierarchical porous structures were composed of large pores having a mean size of 400 μm with interconnected micro-pores in the size of 10–30 μm, where the pore walls possessed numerous nano-pores with a size range of 10–50 nm. The CS and elastic modulus values were 72.4 MPa and 2.67 GPa as well as 92.62 MPa and 3.36 GPa for Ti and TiMo, respectively. The hierarchical porous structure is beneficial for the fast nucleation of bone-like apatite after immersion in simulated body fluid (SBF). In addition, TiMo samples after NaOH and heat treatments provide better apatite formation after soaking in SBF for a week, in comparison with the samples without treatment.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 19: Focus Issue: Porous Metals: From Nano to Macro , 14 October 2020 , pp. 2597 - 2609
- Copyright
- Copyright © Materials Research Society 2020
References
- 1
- Cited by





