Abstract
In the present work, a series of bisbenzazole derivatives were designed and synthesized as antiproliferative agents. The antiproliferative activity of these compounds was investigated using MTT assay. Bisbenzazole derivatives showed significant antiproliferative activity against all the four tested cancer cell lines. Among the various bisbenzazole derivatives, bisbenzoxazole derivatives exhibited the most promising anticancer activity followed by bisbenzimidazole and bisbenzothiazole derivatives. All the derivatives were found to be less toxic as compared to methotrexate (positive control) in normal human cells, indicating selective and efficient antiproliferative activity of these bisbenzazole derivatives. The structure–activity relationships of heteroaromatic systems and linkers present in bisbenzazole derivatives were analyzed in detail. In silico ADMET prediction revealed that bisbenzazole is a drug-like small molecule with a favorable safety profile. Compound 31 is a potential antiproliferative hit compound that exhibits unique cytotoxic activity distinct from methotrexate.
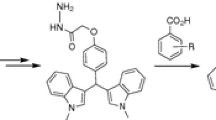
Graphic abstract
Twenty-one bisbenzoxazole derivatives have been designed synthesized and evaluated to be an antiproliferative activity against four human tumor cell lines.






Similar content being viewed by others
References
Spasov AA, Yozhitsa IN, Bugaeva LI (1999) Benzimidazole derivatives: spectrum of pharmacological activity and toxicological properties. Pharm Chem J 33:232–243. https://doi.org/10.1007/BF02510042
Gupta M, Paul S, Gupta R (2009) General characteristics and applications of microwaves in organic synthesis. Acta Chim Slov 56:749–764
Piscitelli F, Ballatore C, Smith A (2010) Solid phase synthesis of 2-aminobenzothiazoles. Bioorg Med Chem Lett 20:644–648. https://doi.org/10.1016/j.bmcl.2009.11.055
Balakumar C, Kishore DP, Rao KV, Narayana BL, Rajwinder K, Rajkumar V, Rao AR (2012) Design, microwave-assisted synthesis and in silico docking studies of new 4H-pyrimido[2,1-b]benzothiazole-2-arylamino-3-cyano-4-ones as possible adenosine A2B receptor antagonists. Indian J Chem 51B:1105–1113
Patil A, Ganguly S, Surana S (2008) A systematic review of benzimidazole derivatives as an antiulcer agent. RJC 1:447–460
Narasimhan B, Sharma D, Kumar P (2012) Benzimidazole: a medicinally important heterocyclic moiety. Med Chem Res 21:269–283. https://doi.org/10.1007/s00044-010-9533-9
Kohli P, Srivastava SD, Srivastava SK (2007) Synthesis and biological activity of mercaptobenzoxazole based thiazolidinones and their arylidenes. J Chin Chem Soc 54:1003–1010. https://doi.org/10.1002/jccs.200700144
Sivakumar R, Pradeepchandran R, Jayaveera KN, Kumarnallasivan P, Vijaianand PR, Venkatnarayanan R (2011) Benzimidazole: an attractive pharmacophore in medicinal chemistry. Int J Pharm Sci Res 3:19–31
Deb PK, Kaur R, Chandrasekaran B, Bala M, Gill D, Kaki VR, Akkinepalli RR, Mailavaram R (2014) Synthesis, anti-inflammatory evaluation, and docking studies of some new thiazole derivatives. Med Chem Res 23:2780–2792. https://doi.org/10.1007/s00044-013-0861-4
Palmer FJ, Trigg RB, Warrington JV (1971) Benzothiazolines as antituberculous agents. J Med Chem 14:248–251. https://doi.org/10.1021/jm00285a022
Hadden MK, Blagg BS (2008) Dimeric approaches to anti-cancer chemotherapeutics. Anticancer Agents Med Chem 8:807–816. https://doi.org/10.2174/187152008785914743
Ueki M, Ueno K, Miyadoh S, Abe K, Shibata K, Taniguchi M, Oi S (1993) A novel cytotoxic metabolite from Streptomyces sp. 517-02. J Antibiot 46:1089–1094. https://doi.org/10.7164/antibiotics.46.1089
Sato S, Kajiura T, Noguchi M, Takehana K, Kobayashi T, Tsuji T (2001) A new cytotoxic benzoxazole derivative produced by Streptomyces sp. J Antibiot 54:102–104. https://doi.org/10.7164/antibiotics.54.102
Jenkins TC (2000) Targeting multi-stranded DNA structures. Curr Med Chem 7:99–115. https://doi.org/10.2174/0929867003375551
Singh MP, Joseph T, Kumar S, Bathini Y, Lown JW (1992) Synthesis and sequence-specific DNA binding of a topoisomerase inhibitory analog of Hoechst 33258 designed for altered base and sequence recognition. Chem Res Toxicol 5:597–607. https://doi.org/10.1021/tx00029a003
Racane L, Kraljevic PS, Ratkaj I, Stepanic V, Pavelic K, Tralic-Kulenovic V, Karminski-Zamola G (2012) Synthesis and antiproliferative evaluation of some new amidino-substituted bis-benzothiazolyl-pyridines and pyrazine. Eur J Med Chem 55:108–116. https://doi.org/10.1016/j.ejmech.2012.07.005
Kumbhare RM, Dadmal T, Kosurkar U, Sridhar V, Rao JV (2012) Synthesis and cytotoxic evaluation of thiourea and N-bis-benzothiazole derivatives: a novel class of cytotoxic agents. Bioorg Med Chem Lett 22:453–455. https://doi.org/10.1016/j.bmcl.2011.10.106
Gravatt GL, Baguley BC, Wilson WR, Denny WA (1994) DNA-directed alkylating agents. 6. Synthesis and antitumor activity of DNA minor groove-targeted aniline mustard analogs of pibenzimol (Hoechst 33258). J Med Chem 37:4338–4345. https://doi.org/10.1021/jm00051a010
Shi Z, Zhao D, Huang Y, Du Y, Cao X, Gong Z, Zhao R, Li J (2012) Discovery, synthesis, and evaluation of small-molecule signal transducer and activator of transcription 3 inhibitors. Chem Pharm Bull 60:1574–1580. https://doi.org/10.1248/cpb.c12-00745
McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR (1995) The end of the (cell) line: methods for the study of apoptosis in vitro. Methods in Cell Biology: Cell Death 46:153–185. https://doi.org/10.1016/s0091-679x(08)61929-9
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
van Meerloo J, Kaspers GJL, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245. https://doi.org/10.1007/978-1-61779-080-5_20
Lan P, Romero FA, Wodka D, Kassick AJ, Dang Q, Gibson T, Cashion D, Zhou G, Chen Y, Zhang X, Zhang A, Li Y, Trujillo ME, Shao Q, Wu M, Xu S, He H, MacKenna D, Staunton J, Chapman KT, Weber A, Sebhat IK, Makara GM (2017) Hit-to-lead optimization and discovery of 5-((5-([1, 1′-Biphenyl]-4-yl)-6-chloro-1 H-benzo [d] imidazol-2-yl) oxy)-2-methylbenzoic Acid (MK-3903): A novel class of benzimidazole-based activators of AMP-activated protein kinase. J Med Chem 60:9040–9052. https://doi.org/10.1021/acs.jmedchem.7b01344
Elagab HA, Alt HG (2015) Structure–property-relationship studies with ethylene polymerization catalysts of Ti, Zr and V containing heterocyclic ligands. Inorgan Chim Acta 437:26–35. https://doi.org/10.1016/j.ica.2015.08.002
Qian J, Zhang Y, Yin X (2012) The synthesis and study of bis(1-octylbenzimidazoi-2-yl)alkane oil-soluble corrosion inhibitor. Huaxue Tongbao 75:88–91
Heinrich J, Koenig NF, Sobottka S, Sarkar B, Kulak N (2019) Flexible vs. rigid bis(2-benzimidazolyl) ligands in Cu(II) complexes: impact on redox chemistry and oxidative DNA cleavage activity. J Inorg Biochem 194:223–232. https://doi.org/10.1016/j.jinorgbio.2019.01.016
Yilmaz U, Kucukbay H (2016) Synthesis and characterization of novel phosphoramidates containing benzimidazole moiety. Phosphorus, Sulfur Silicon Relat Elem 191:140–143. https://doi.org/10.1080/10426507.2015.1067209
Inamdar SM, More VK, Mandal SK (2013) CuO nano-particles supported on silica, a new catalyst for facile synthesis of benzimidazoles, benzothiazoles and benzoxazoles. Tetrahedron Lett 54:579–583. https://doi.org/10.1016/j.tetlet.2012.11.091
Berends HP, Stephan DW (1984) Copper (I) and copper (II) complexes of biologically relevant tridentate ligands. Inorgan Chim Acta 93:173–178. https://doi.org/10.1016/S0020-1693(00)88159-1
Mao S, Shen K, Shi X, Wu H, Han X, Li C, Huang G (2018) Synthesis, crystal structure and biological activity of two binuclear Ag(I) complexes with bis-benzimidazole thioether ligands. Inorgan Chim Acta 471:82–90. https://doi.org/10.1016/j.ica.2017.10.038
Rao SS, Reddy CV, Dubey PK (2014) Synthesis of N,N-disubstitutedbisbenzimidazolesulphides of Potential Pharmacological Interest. JCHPS 6:1199–1204
Xu Y, Wu H, Zhang H, Aderinto SO, Yang Z (2016) Synthesis, crystal structures, and DNA-binding studies of two silver (I) complexes with 1, 3-bis(1-ethylbenzimidazol-2-yl)-2-thiapropane. J Coord Chem 69:2988–2998. https://doi.org/10.1080/00958972.2016.1218484
Mao S, Shen K, Shi X, Xu Y, Wu H (2017) Two silver (I) complexes with bis (benzimidazole)-2-oxopropane ligands: syntheses, crystal structures and DNA binding studies. APPl 31:e3747. https://doi.org/10.1002/aoc.3747
Wu HL, Yun RR, Wang KT, Li K, Huang XC, Sun T (2010) Synthesis, crystal structure, and spectrum properties of Cobalt (II) complexes based on tridentate 1, 3-bis(benzimidazol-2-yl)-2-oxopropane ligand and derivative. Z Anorg Allg Chem 636:629–633. https://doi.org/10.1002/zaac.200900298
Feng R, Hou Y, Wu Z, Yang Y, Nie F (2015) Structures and fluorescent properties of Cadmium (II) complexes with 1D and 2D Structures based on tridentate benzimidazole ligands. Z Anorg Allg Chem 641:1918–1925. https://doi.org/10.1002/zaac.201400511
Kopel P, Wawrzak D, Langer V, Cihalova K, Chudobova D, Vesely R, Adam V, Kizek R (2015) Biological activity and molecular structures of bis(benzimidazole) and trithiocyanurate complexes. Molecules 20:10360–10376. https://doi.org/10.3390/molecules200610360
Chen B, Morlanes N, Adogla E, Takanabe K, Rodionov VO (2016) An efficient and stable hydrophobic molecular cobalt catalyst for water electro-oxidation at neutral pH. ACS Catal 6:4647–4652. https://doi.org/10.1021/acscatal.6b01237
Dauer D, Stalke D (2014) Heterocyclic substituted methanides as promising alternatives to the ubiquitous nacnac ligand. Dalton Trans 43:14432–14439. https://doi.org/10.1039/C4DT01008F
Kretsch J, Kreyenschmidt A, Herbst-Irmer R, Stalke D (2018) Alkali metal complexes based on bisheterocyclomethanide ligands. Dalton Trans 47:12606–12612. https://doi.org/10.1039/C8DT01678J
Kumar R, Selvam C, Kaur G, Chakraborti AK (2005) Microwave-assisted direct synthesis of 2-substituted benzoxazoles from carboxylic acids under catalyst and solvent-free conditions. Synlett 9:1401–1404. https://doi.org/10.1055/s-2005-868509
Shirakawa Y, Masuda T (2013) Coupling agents for rubber/carbon black and rubber compositions containing them. PCT Int Appl WO 2013015425
Cakir B, Ucucu U, Buyukbingol E, Abbasoglu U (1989) Benzoxazoles: bis-benzoxazole derivatives, synthesis, antifungal activities and QSARs. J Faculty Pharm Gazi Uni 6:15–21
Terao H, Ono Y, Ito Y, Isogai M, Hamada T, Imanishi, Tsunoda A (1991) Organic nonlinear optical device. Japanese Kokai Tokkyo Koho JP 03188425
Hao Y, Chen Y (2016) Excited-state intramolecular single and double proton transfer emission of 2,5-bis(benzoxazol-2-yl) thiophene-3,4-diol. Dyes Pigme 129:186–190. https://doi.org/10.1016/j.dyepig.2016.03.002
Hao Y, Zheng M, Chen Y (2014) A highly stable and water-soluble fluorescent dye for fluorescence imaging of living cells. J Mater Chem B 2:7369–7374. https://doi.org/10.1039/C4TB01210K
Zhou H, Wang L, Yin B (2004) Synthesis of bis(2-benzoxazolylmethyl) ether by dry reaction under microwave irradiation. Huaxue Shiji 26:308–311
Alt H, Elagab H, Al-Humydi A (2011) Ethylene Polymerisation. PCT Int Appl WO 2011088990
Chakraborti AK, Selvam C, Kaur G, Bhagat S (2004) An efficient synthesis of benzothiazoles by direct condensation of carboxylic acids with 2-aminothiophenol under microwave irradiation. Synlett 5:851–855. https://doi.org/10.1055/s-2004-820012
Katritzky AR, Liang D, Fan W (1988) Bridged cyanine dyes. Part 2 [1].1-(N-methyl-2-benzothiazolylinio)-3-(N-methyl-2-benzothiazolylene) and 1-(N-methyl-4-pyridinio)-3-(N-methyl-4-pyridylene)cyclopenta-1,4-dienes with fused rings. J Heterocycl Chem 25:1315–1319. https://doi.org/10.1002/jhet.5570250509
Rai C, Braunwarth JB (1961) Synthesis of bisbenzothiazoles1. J Org Chem 26:3434–3436. https://doi.org/10.1021/jo01067a100
Strasser CE, Jongh LAD, Raubenheimer HG, Cronje S (2011) 2,2′-(Sulfanediyldimethylene)bis(1,3-benzothiazole). Acta Cryst E 67:o622. https://doi.org/10.1107/S1600536811004478
Sih JC, Graber DR (1983) 2-Mercapto-1,3-benzoxazole: a useful reagent for the preparation of symmetrical and unsymmetrical sulphides. J Org Chem 48:3842–3845. https://doi.org/10.1021/jo00169a058
Das SK, Mathur P (1999) Copper (II) complexes with bis thiazole based ligands: spectral, cyclic voltammetric and EPR studies. Indian J Chem A 38A:1277–1282
Ushenko IK (1952) α,γ-Epoxythiacarbocyanines. I Zh Obshch Khim 22:711–715
Finn MG, Rodionov VO (2009) Ligands for copper-catalyzed azide-alkyne cycloaddition reactions. PCT Int Appl WO 2009038685
Buehrdel G, Beckert R, Herzigova P, Petrlikova E, Schuch D, Birckner E, Goerls H (2009) A new synthesis of push–pull pyrroles, their oxidation to stable 3H-pyrroles and an unexpected anellation reaction. Eur J Org Chem 20:3404–3412. https://doi.org/10.1002/ejoc.200900295
Akpa SJ, Say MV, Zoakouma SPR, Fante B, Sissouma D, Adjou A (2016) Synthesis of 2-(benzylthio) benzimidazole, 2-[(benzimidazol-2-yl)methylthio]benzimidazole and structural analogues against Haemoncus contortus. AFR J Pharm Pharmacol 10:670–680. https://doi.org/10.5897/AJPP2016.4557
Bouchouit M, Said M, Kara M, Bouacida S, Merazig H, Kacem-Chaouche N, Chibani A, Zouchoune B, Belfaitah A, Bouraiou A (2016) Synthesis, X-ray structure, theoretical investigation, corrosion inhibition and antimicrobial activity of benzimidazole thioether and theirs metal complexes. Polyhedron 119:248–259. https://doi.org/10.1016/j.poly.2016.08.045
Zubarovskii VM (1951) 2-(Hydroxymethyl)benzothiazole and its transformations. Zh Obshch Khim 21:2055–2064
Sabina XJ, Karthikeyan J, Velmurugan G, Tamizh MM, Shettyd AN (2017) Design and in vitro biological evaluation of substituted chalcones synthesized from nitrogen mustards as potent microtubule targeted anticancer agents. New J Chem 41:4096–4109. https://doi.org/10.1039/C7NJ00265C
Acknowledgements
We thank the Scientific and Technological Research Council of Turkey (TUBITAK, Grant Number: 115S190) and Mersin University (Project Number: 2018-1-TP3-2911) for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ersan, R.H., Alagoz, M.A., Ertan-Bolelli, T. et al. Head-to-head bisbenzazole derivatives as antiproliferative agents: design, synthesis, in vitro activity, and SAR analysis. Mol Divers 25, 2247–2259 (2021). https://doi.org/10.1007/s11030-020-10115-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10115-0