Abstract
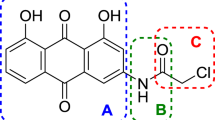
N-alkylated and O-alkylated anthraquinone derivatives with structures analogous to mitoxantrone were synthesized, characterized, and evaluated for their cytotoxic properties against three tumor cell lines (Human Breast Adenocarcinoma MCF-7, Human Cervical Adenocarcinoma HeLa, and Human Glioblastoma M059J) and a normal cell line (human lung fibroblasts GM-07492A). A structure-activity relationship study was carried out to verify the influence of lipophilic chain size on the biological activity of these compounds. The results indicated promising candidates for antineoplastic agents for the cancers evaluated, since these compounds showed significant selectivity and high cytotoxic potential for cancer cells, rather than mitoxantrone, the compound of which is already used in anticancer therapy.


Similar content being viewed by others
References
Alves CCS, Castro SBR, Costa CF, Dias AT, Alves CJ, Rodrigues MF, Teixeira HC, De Almeida MV, Ferreira AP (2012) Anthraquinone derivative O, O′-bis-(3′-iodopropyl)-1, 4-dihidroxyanthraquinone modulates immune response and improves experimental autoimmune encephalomyelitis. Int Immunopharmacol 14:127–132
Alves CCS, Collison A, Hatchwell L, Plank M, Morten M, Foster PS, Johnston SL, Da Costa CF, De Almeida MV, Teixeira HC, Ferreira AP, Mattes J (2013) Inhibiting AKT phosphorylation employing non-cytotoxic anthraquinones ameliorates TH2 mediated allergic airways disease and rhinovirus exacerbation. PLoS ONE 8:79565
Corrêa TA, Alves CCS, Castro SBR, Oliveira EE, Franco LS, Ferreira AP, Almeida MV (2013) Synthesis of 1,4-anthracene-9,10-dione derivatives and their regulation of nitric oxide, IL-1β and TNF-α in activated RAW264.7 cells. Chem Biol Drug Des 82:463–467
Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JAP, Saffi J (2016) Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch toxicol 90:2063–2076
Gareth G (2010) Química medicinal: Uma introdução. Guanabara, Rio de Janeiro
Huang HS, Chiu HF, Lee AL, Guo CL, Yuan CL (2004) Synthesis and structure–activity correlations of the cytotoxic bifunctional 1,4-diamidoanthraquinone derivatives. Bioorg Med Chem 12:6163–6170
Johnson MG, Kiyokawa H, Tani S, Koyama J, Morris-Natschke SL, Mauger A, Bowers-Daines MM, Lange BC, Lee KH (1997) Antitumor Agents-CLXVII. Synthesis and structure-activity correlations of the 124 cytotoxic anthraquinone 1,4-bis-(2,3-epoxypropylamino)-9,10-anthracenedione, and of related compounds. Bioorg Med Chem 5:1469–1479
Jones JL, Anderson JM, Phuah PL, Fox EJ, Selmaj K, Margolin D, Lake SL, Palmer J, Thompson SJ, Wilkins A, Webber DJ, Compston DA, Coles AJ (2010) Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 133:2232–4227
Krapcho AP, Landi Jr JJ, Shaw KJ, Phinney DG, Hacker MP, McCormack JJ (1986) Synthesis and antitumor activities of unsymmetrically substituted 1,4-Bis[(aminoalkyl)amino]anthracene-9,10-dioneas and related systems. J Med Chem 29:1370–1373
Kumar PH, Prakash SS, Kumar SK, Diwakar L, Reddy GC (2011) Synthesis of mitoxantrone analogues and their in-vitro cytotoxicity. Int J Chem Tech 3:690–694
Lytle NK, Barber AG, Reya T (2018) Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer 18:669–680
Neuhaus O, Kieseier BC, Hartung HP (2006) Therapeutic role of mitoxantrone in multiple sclerosis. Pharmacol Ther 109:198–209
Salustiano EJ, Dumas ML, Silva-Santos GG, Netto CD, Costa PRR, Rumjanek VM (2016) In vitro and in vivo antineoplastic and immunological effects of pterocarpanquinone LQB-118. Investig N Drugs 34:541–51
Shcekotikhin AE, Glazunova VA, Dezhenkova LG, Luzikov YN, Sinkevich YB, Kovalenko LV, Buyanov VN, Balzarini J, Huang FC, Lin JJ, Huang HS, Shtil AA, Preobrazhenskay MN (2009) Synthesis and cytotoxic properties of 4,11-bis[(aminoethyl)amino]anthra-[2,3-b]thiophene-5,10-diones, novel analogues of antitumor anthracene-9,10-diones. Bioorg Med Chem 17:1861–1869
Suffness M, Pezzuto JM (1990) Assays related to cancer drug discovery. In:Hostettmann K (ed) Methods in plant biochemistry: assay for bioactivity. Academic Press, London, p 71–133
Ulrich SH AM T, Hans PK, Hans-Harald S, Hoechst A (1998) Monofunctional and bisfunctional anthraquinone-(oxy-2,3-oxidopro panes), processes for their preparation, and their use as drugs. US Patent 4.762.648. 9 Aug 1998
Varadwaj P, Misra K, Sharma A, Kuma R (2010) Mitoxantrone: an agent with promises for anticancer therapies. Electron J Biol 6:36–42
Acknowledgements
This work was supported by grants from FAPEMIG, CNPq, CAPES, Rede Mineira de Química, and UFJF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Oliveira, L.A., Nicolella, H.D., Furtado, R.A. et al. Design, synthesis, and antitumor evaluation of novel anthraquinone derivatives. Med Chem Res 29, 1611–1620 (2020). https://doi.org/10.1007/s00044-020-02587-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02587-4