Abstract
Zika virus (ZIKV) and Japanese encephalitis virus (JEV) are closely related to mosquito-borne flaviviruses. Japanese encephalitis (JE) vaccine SA14-14-2 has been in the Chinese national Expanded Program on Immunization since 2007. The recent recognition of severe disease syndromes associated with ZIKV, and the identification of ZIKV from mosquitoes in China, prompts an urgent need to investigate the potential interaction between the two. In this study, we showed that SA14-14-2 is protective against ZIKV infection in mice. JE vaccine SA14-14-2 triggered both Th1 and Th2 cross-reactive immune responses to ZIKV; however, it was cellular immunity that predominantly mediated cross-protection against ZIKV infection. Passive transfer of immune sera did not result in significant cross-protection but did mediate antibody-dependent enhancement in vitro, though this did not have an adverse impact on survival. This study suggests that the SA14-14-2 vaccine can protect against ZIKV through a cross-reactive T cell response. This is vital information in terms of ZIKV prevention or precaution in those ZIKV-affected regions where JEV circulates or SA14-14-2 is in widespread use, and opens a promising avenue to develop a novel bivalent vaccine against both ZIKV and JEV.
Key points
• JEV SA14-14-2 vaccine conferred cross-protection against ZIKV challenge in mice.
• T cell immunity rather than antibody mediated the cross-protection.
• It provides important information in terms of ZIKV prevention or precaution.
Similar content being viewed by others
Introduction
Recently, Zika virus (ZIKV) has caused devastating outbreaks of fetal congenital malformations in South and Central America and now transmitted in more than 70 countries, including many previously unaffected regions. ZIKV infection during pregnancy increases the risk of neurological disorders in newborns (Zhou et al. 2019), such as microcephaly. In adults, ZIKV causes Guillain-Barré syndrome and other neurologic disorders (Mendez et al. 2017). So far, no specific vaccine or antiviral for the prevention and treatment of Zika has been licensed.
ZIKV is a member of the genus Flavivirus, family Flaviviridae, which contains more than 70 viruses. Among them, mosquito-borne flaviviruses such as Japanese encephalitis (JE) virus (JEV), dengue virus (DENV), ZIKV, yellow fever virus (YFV), and West Nile virus pose a threat to half of the world population and cause significant public health impact in many developing countries (Guarner and Hale 2019).
The flavivirus genome consists of non-segmented single-stranded positive-sense RNA, which encodes three structural proteins including the capsid protein (C), the membrane protein (M), and the envelope protein (E), and seven non-structural (NS) proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). Within the same genus, these mosquito-borne flaviviruses are antigenically related to various degrees (Heinz and Stiasny 2017). Among them, JEV, ZIKV, and DENV share more than 50% amino acid sequence identity by pairs (Chang et al. 2017; Strauss and Strauss 2001). On average, ZIKV shares a 55.6% protein sequence identity with DENV and 56.1% with JEV (Chang et al. 2017).
Currently, several studies have indicated complex interactions between DENV and ZIKV immunity (Breitbach et al. 2019; Fowler et al. 2018; Pantoja et al. 2017; Slon-Campos et al. 2019; Wang et al. 2019a; Zimmerman et al. 2018). Clinical data suggest that pre-existing DENV immunity is partially protective against symptomatic ZIKV infection and against congenital ZIKV syndrome (Gordon et al. 2019; Pedroso et al. 2019). Earlier DENV infection also probably partially protects against JE indicating the possibility of a more general effect within the genus (Grossman et al. 1974).
Previously, we demonstrated that JEV SA14-14-4 live attenuated vaccine, an inactivated vaccine based on P3 strain, and a JE DNA vaccine based on the premembrane and E proteins, effectively elicited the production of cross-reactive antibodies, cytokines, and cellular immune responses and generated cross-protection against four serotypes of DENV (Gao et al. 2019; Li et al. 2016). However, little is known about cross-reactivity between JEV and ZIKV. The geographic overlap, possibility of sequential infection with JEV and ZIKV, and widespread use of the JEV SA14-14-2 vaccine in China indicate a need to understand the impact of pre-existing immunity to JEV (acquired through either SA14-14-2 vaccination or natural infection) on ZIKV infection. Therefore, in this study, we aimed to evaluate the cross-reactivity and cross-protection of JEV SA-14-14-2 vaccination against ZIKV infection in a mouse model. Our findings suggest cross-immunity between the JE vaccine and ZIKV and indicate a need for further study in humans to address the role of JE immunity in protection from ZIKV.
Materials and methods
Cells, viruses, vaccine, and mice
C6/36 cells (ATCC™ number CRL-1660) were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) at 28 °C. Vero cells (ATCC™ number CCL-81) were cultured in minimal essential medium (MEM, Gibco, USA) supplemented with 5% FBS at 37 °C. THP-1 cells (ATCC™ number TIB-202) were cultured in RPMI 1640 medium supplemented with 10% FBS at 37 °C. All cells were cultivated under a humidified atmosphere of 5% CO2.
The JEV (Beijing-1 strain, GenBank accession number L48961.1) and the ZIKV (SMGC-1 strain, GenBank accession number KX266255) were propagated in C6/36 cell cultures and stored at − 80 °C. JEV was kindly provided by Dr. Kotaro Yasui (Department of Microbiology, Tokyo Metropolitan Institute for Neuroscience, Tokyo, Japan). ZIKV was kindly provided by Dr. George F. Gao (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China). Virus titers were determined by plaque assay on Vero cells under MEM with 1.2% methylcellulose overlay medium. Propagated virus was harvested from the supernatant of C6/36 cells infected with the virus, concentrated by 8% polyethylene glycol precipitation, and purified from clarified extracts by ultracentrifugation. The JE live attenuated vaccine (SA14-14-2 strain) was produced by the Chengdu Institute of Biological Products (China).
Female and male C57BL/6 mice and female Ifnar1-/- mice were housed under specific pathogen-free conditions, and C57BL/6 mice were bred to obtain neonatal mice. Adult female mice were used at 6 weeks of age, and neonatal Ifnar1-/- mice were used between 24 h and 36 h after birth.
Mouse immunization
Six-week-old female adult mice were divided randomly into vaccine and control groups. C57BL/6 and Ifnar1-/- mice in the vaccine group were immunized intraperitoneally (i.p.) with 104 and 103 plaque-forming units (PFU) JEV SA14-14-2 strain, respectively, three times at 3-week intervals (Li et al. 2016). Control mice were injected with PBS following an identical schedule.
Cross-reactive protection against ZIKV in SA14-14-2-immunized Ifnar1-/- mice
Three weeks after the final vaccination, at 15 weeks of age, the Ifnar1-/- mice were challenged i.p. with a lethal dose of JEV or ZIKV (103 PFU for both). Body weight and mortality were monitored daily for 14 consecutive days.
Plaque reduction neutralization test
Sera were collected from the C57BL/6 mice 3 weeks after the final vaccination. Neutralizing antibody (nAb) titers were detected by measuring plaque reduction neutralization titer (PRNT) as previously reported (Wang et al. 2018, 2019b). Serum samples were heated at 56 °C for 30 min to inactivate complement and then twofold serially diluted from 1:10 to 1:1280. Diluted sera were mixed 1:1 with virus suspension containing 50 plaque-forming units (PFU) and incubated at 37 °C for 1 h. The mixture was transferred to a confluent monolayer of Vero cells in a 24-well plate and incubated at 37 °C for another 1 h. After washing, the infected Vero cells were overlaid with MEM containing 1.2% methylcellulose followed by incubation at 37 °C for 5 to 8 days. Plaques were visualized by crystal violet counterstaining and counted. The reciprocal highest serum dilution yielding a 50% reduction of the average number of plaques as compared with the virus infection wells was calculated as the 50% neutralization titer (PRNT50).
In vitro neutralizing and passive cross-protective effects of immune sera in neonatal C57BL/6 mice
Sera were collected from C57BL/6 mice 3 weeks after the final vaccination. After heat inactivation, pooled sera were mixed with either live JEV (Beijing-1 strain) or the ZIKV (SMGC-1 strain) followed by incubation at 37 °C for 1 h. Subsequently, 10 μl of serum/virus mixture containing 150 PFU of virus was gently injected intracerebally (i.c.) into neonatal C57BL/6 mice. The mice were monitored daily for body weight and mortality for 31 consecutive days.
Enzyme-linked immunosorbent assay
To detect IgG antibodies and their subclasses, ELISA was performed according to the method as previously described (Wang et al. 2018). Sera were collected from C57BL/6 mice 3 weeks after the final vaccination. Each well of 96-well plates was coated with purified viral particles (105 PFU) from JEV or ZIKV and then blocked with 3% bovine serum albumin. Sera from immunized mice were twofold serially diluted in PBS (from 1:100 to 1:204,800), and IgG antibodies were measured with goat anti-mouse secondary antibodies (1:4000, alkaline phosphatase coupled, Abcam, USA) and substrate solution of p-nitrophenyl phosphate (Sigma, USA). The optical density (O.D.) at 405 nm was measured using an ELISA reader (Thermo, USA). The reciprocal of the highest dilution that yielded the O.D. value greater than half of the O.D. value of corresponding control at 1:100 dilution was recorded as the endpoint titer of IgG antibody (Wang et al. 2018).
To determine IgG subclasses, mouse sera (at 1:100 dilution) were used as the primary antibodies; IgG1, IgG2a, IgG2b, and IgG3 subsets were detected with goat anti-mouse secondary antibodies (1:4000, alkaline phosphatase coupled, Abcam, USA) and substrate solution of p-nitrophenyl phosphate; and the levels of IgG subclasses were recorded as O.D. value at 405 nm.
Antibody-dependent enhancement assay
Three weeks after the final immunization, sera were collected from C57BL/6 mice. Serially tenfold diluted sera were incubated with JEV or ZIKV at a multiplicity of infection of 2 for 1 h at 37 °C before adding to THP-1 cells. Samples were incubated for 2 h at 37 °C and gently shaken every 15 min. Cells were centrifuged and washed three times, followed by resuspension in fresh RPMI 1640 medium and incubated for 3 days at 37 °C. Supernatants were collected, and viral titer measured by plaque assay on Vero cells as described (Wang et al. 2018). Fold enhancement was calculated by comparison with viral titers in the absence of immune sera.
Enzyme-linked immunospot assays
Three weeks after the final immunization, the cytokines IL-2, IL-4, and IFN-γ secreted by the splenocytes of C57BL/6 mice were determined using enzyme-linked immunospot (ELISPOT) kits (BD, USA) according to the manufacturer’s instructions and the previous protocol (Wang et al. 2020, 2019c). In brief, mouse splenocytes were plated at 3 × 105/well into 96-well filtration plates (Millipore, USA) pre-coated with capture antibodies and stimulated with 5 μg/well purified JEV or ZIKV particles for 60 h at 37 °C. After incubation with biotinylated detection antibody, the spots were visualized by adding streptavidin-horseradish peroxidase and 3-amino-9-ethylcarbazole substrate, counted automatically with an ELISPOT reader (CTL, USA) and analyzed by ImmunoSpot software (version 5.1). Splenocytes cocultured with concanavalin A served as a positive control, and those cultured with RPMI 1640 medium served as a negative control.
Adoptive transfer and cross-reactive protection of splenocytes from SA14-14-2-immunized C57BL/6 mice in Ifnar1-/- mice
Three weeks after the final vaccination, splenocytes were isolated from the immunized C57BL/6 mice using lymphocyte separation Percoll (Solarbio, China). A total of 3 × 106 lymphocytes was transfused retro-orbitally (r.o.) to female adult Ifnar1-/- mice. After 24 h, the mice were challenged i.c. with a lethal dose of either JEV (Beijing-1 strain, 104 PFU) or ZIKV (SMGC-1 strain, 104 PFU). Body weight and mortality were monitored daily for 14 consecutive days. Mice exhibiting more than 25% loss in body weight were humanely euthanized for ethical reasons.
Statistical analysis
Statistical analyses were performed using SPSS statistics version 17.0 (SPSS Inc., USA). Geometric mean titers (GMTs) were calculated after the log transformation of reciprocal titers. Weight changes were analyzed by repeated measures analysis of variance. Kaplan-Meier survival curves were plotted and evaluated statistically by the log-rank test. Others were analyzed using one-way analysis of variance. The results were presented as means +/−/± standard deviation (SD), and the difference between means is considered significant if P < 0.05. P values are denoted with an asterisk if P < 0.05, double asterisk if P < 0.01, and triple asterisk if P < 0.001, respectively.
Results
Cross-protection against lethal ZIKV challenge in immunocompromised mice
A number of studies have used Ifnar1-/- mice to evaluate the effectiveness of vaccines despite the immunodeficiency of the mice (Lecouturier et al. 2019; Shan et al. 2019). Although Ifnar1-/- mice lack the innate type 1 IFN response, they retain adaptive immunity but are highly susceptible to ZIKV (Dowall et al. 2016), representing a suitable model for testing vaccine-induced cross-protective adaptive immunity. First, we used this model to determine whether JEV SA14-14-2 has a cross-protective effect on ZIKV. Three doses of JE vaccine SA14-14-2 were administrated to Ifnar1-/- mice, followed by challenge with JEV or ZIKV 3 weeks post the final immunization (Fig. 1a). As expected, after JEV challenge, all mice inoculated with JEV SA14-14-2 survived (6/6) during the observation period without any weight loss (Fig. 1b, c). In contrast, the control mice showed a rapid decrease 1 day post JEV challenge and all mice died by the humane endpoint on day 3 post challenge. Interestingly, SA14-14-2-vaccinated Ifnar1-/- mice were completely protected (6/6) against ZIKV infection, as compared with PBS-treated mice where 83.3% (5/6) mice succumbed to infection (Fig. 1b, c). Vaccinated mice maintained a normal body weight whereas control mice showed significant weight loss (approximately 16%) and most of them died within 9 days (1/6 survival). These data suggest that vaccination with SA14-14-2 in Ifnar1-/- mice induced in vivo cross-reactive immunity, conferring effective cross-protection against lethal ZIKV infection.
Cross-reactive protection against ZIKV in SA14-14-2-immunized Ifnar1-/- mice. a Schedule. Female Ifnar1-/- mice were immunized three times at 3-week intervals. Three weeks after the final vaccination, the Ifnar1-/- mice were challenged i.c. with a lethal dose of JEV or ZIKV. Body weight and mortality were monitored daily for 14 consecutive days. b Percentage changes in body weight. Data were expressed as mean ± SD. c Survival rate was shown as the percentage of survivors (n = 6). Mice exhibiting more than 25% loss in weight were humanely euthanized for ethical reasons. Each experiment was independently repeated three times. Asterisk indicates P < 0.05; double asterisk indicates P < 0.01; triple asterisk indicates P < 0.001
Cross-protection against ZIKV infection is not mediated by SA14-14-2 immune sera
There is a clearly established role for nAb in protection from flaviviruses. Therefore, in order to evaluate cross-reactive neutralization of ZIKV induced by SA14-14-2 vaccination, 3 weeks after the final immunization, sera were collected from C57BL/6 mice and the nAb titers were assayed by PRNT (Fig. 2a). As expected, mice administered three doses of JEV SA14-14-2 developed a high level of JEV-specific nAb, with a GMT of 1:494 (Fig. 2b). In contrast, JEV SA14-14-2 antisera from the immunized mice failed to neutralize ZIKV, and the PRNT50 titer of antisera was comparable to that of controls. This result suggests that nAb elicited by JEV SA14-14-2 yielded no neutralizing activity against ZIKV.
Cross-reactive nAb responses in mouse sera. a Schedule. Female adult C57BL/6 mice were immunized as previously described. Sera were collected and mixed with either JEV (Beijing-1 strain) or ZIKV (SMGC-1 strain). Then, serum/virus mixture was gently injected i.c. into neonatal C57BL/6 mice. b Serum cross-reactive nAb titers assayed by PRNT50 (n = 7). NAb titers are expressed as GMT + SD. c, d In vitro neutralizing and passive cross-protective effects of immune sera (n = 8, 8, 10, 12, respectively). The mice were monitored daily for body weight and survival rate for 31 consecutive days. c Body weight was expressed as mean ± SD. d Survival rate was shown as the percentage of survivors. Triple asterisk indicates P < 0.001; NS, non-significant
To further test whether JEV SA14-14-2 antisera would cross-protect mice against ZIKV challenge in vitro, for example, by a mechanism distinct from neutralization, immune sera were harvested from SA14-14-2-vaccinated C57BL/6 mice on week 3 after the final immunization. A serum/virus (JEV or ZIKV) mixture was prepared and inoculated i.c. into naïve neonatal C57BL/6 mice (Fig. 2a). Control sera from C57BL/6 mice injected with PBS were included in the same experiment. SA14-14-2 immune serum protected neonatal mice (8/8) from JEV challenge, after which the mice exhibited steady growth and development, manifesting a normal and continuous weight increase, reaching 15.4 g at the end of observation (Fig. 2c, d). In contrast, none of the neonatal mice (0/8) receiving non-immune sera survived JEV challenge and exhibited severe growth delay, with an endpoint weight of only 2.0 g. ZIKV challenge was less pathogenic in this model than JEV, with control mice (ZIKV infected receiving no sera) developing subnormally but with indistinctive body weight change (Fig. 2c). No effect of SA14-14-2 immune serum could be detected in this experiment, and the terminal weights of the mice receiving JEV SA14-14-2 immune serum and control mice were 9.8 g and 9.1 g, respectively. Survival in the two groups was also the same, 10.0% (1/10) in mice incoculated with a mixture of SA14-14-2 immune sera and ZIKV and 8.3% in mice injected with control mixture (1/12, Fig. 2c, d). Although SA14-14-2 immune sera did slightly extend the median survival of neonatal ZIKV-challenged mice (22.0 days vs. 15.0 days), this effect was not significant by the log-rank test. These data suggest a potent JEV-specific protective effect of SA14-14-2 immune sera, but no cross-protection from ZIKV infection, consistent with the nAb data.
Presence of cross-reactive IgG antibody and its multiple subclasses in response to ZIKV induced by SA14-14-2 vaccination
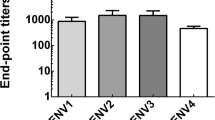
While we could detect no protective role of cross-reactive antibody against ZIKV infection from SA14-14-2 immune sera, we sought to determine whether there was any binding to ZIKV, as cross-reactive binding but non-neutralizing antibodies have been described (Dejnirattisai et al. 2016). Thus, to determine the presence of JEV-specific and ZIKV cross-reactive IgG antibody and its subclasses, including IgG1, IgG2a, IgG2b, and IgG3, induced by SA14-14-2 vaccination, sera were collected 3 weeks after the last immunization from C57BL/6 mice and analyzed by ELISA (Fig. 3a). Levels of both JEV-specific and ZIKV cross-reactive IgG antibodies were higher in the sera of immunized mice than those in corresponding controls (1:60,887 vs. 1:519 and 1:1345 vs. 1:436 endpoint titers, respectively, Fig. 3b), suggesting the induction of a cross-reactive humoral immune response to ZIKV. Furthermore, to informatively characterize the profile of cross-reactive IgG antibodies induced by SA14-14-2-vaccination, IgG1, IgG2a, IgG2b, and IgG3 subclasses in immune sera were measured (Fig. 3c). It is known that isotype switching to IgG1 is promoted by a Th2 response, whereas switching to IgG2a, IgG2b, and IgG3 is promoted by a Th1 response (Germann et al. 1995). Our data show that IgG1, IgG2a, and IgG2b were all detected after vaccination, but IgG3 was not induced. These data suggest that SA14-14-2 vaccination could induce a response with both Th1 and Th2 components.
Cross-reactive IgG and its subclass responses and ADE in mouse sera. a Schedule of mouse immunization and serum collection. Female adult C57BL/6 mice were immunized three times at 3-week intervals. Sera were collected 3 weeks after the final immunization. b Cross-reactive IgG responses detected by ELISA (n = 7). Ab titers are recorded as GMT + SD. c Cross-reactive IgG subclass responses determined by ELISA (n = 7). d ADE in sera measured by plaque forming (n = 7 per dilution). Asterisk indicates P < 0.05; double asterisk indicates P < 0.01; triple asterisk indicates P < 0.001
SA14-14-2 immune sera induce antibody-dependent enhancement in vitro
Antibody-dependent enhancement (ADE) occurs when the titer or neutralization potential of serum is too low to achieve complete neutralization, but the antibody is still able to bind to the virus, which then promotes entry into Fc receptor–bearing cells, which are permissive for viral replication. The effect is common among flaviviruses (Langerak et al. 2019). Therefore, in order to determine whether the SA14-14-2 immune sera could promote ADE of ZIKV infectivity in vitro, we exposed FcγRI/II-bearing cell line THP-1 to ZIKV in the presence or absence of SA14-14-2 immune sera. We observed dose-dependent enhancement of infection from a dilution of 1:100, which peaked at 1:10,000 dilution, with ZIKV infection enhancement up to 79.7-fold (Fig. 3d). In contrast, sera from control mice did not significantly enhance the infectivity of ZIKV, although modest enhancement at a dilution of 1:10,000, likely due to non-specific effect. Meanwhile, when THP-1 cells were infected in the presence of the SA14-14-2 immune sera, we found that they also yielded a 17.0-fold greater infection of JEV at a dilution of 1:10,000 than those in the absence of sera (Fig. 3d). This homotypical enhancement is most likely the result of sub- or non-neutralizing titer of serum dilution. As expected, the control sera did not obviously enhance JEV infection. In summary, the result demonstrates that cross-reactive anti-JEV antibodies can promote ADE of ZIKV, at least in vitro.
Multiple cross-reactive cytokine responses to ZIKV
Having provided indirect evidence that both Th1 and Th2 responses were made following SA14-14-2 vaccination and that these responses could cross-react with ZIKV, albeit not with protective nAb, we sought to determine the nature of the T helper response after SA14-14-2 vaccination. Three weeks after the third and final immunization, splenocytes were collected from the C57BL/6 mice. The levels of splenocyte-derived IL-2, IL-4, and IFN-γ were determined by ELISPOT assay (Fig. 4). Notably, when pulsed with ZIKV antigen, splenocytes from SA-14-14-2-immunized mice responded by making all three cytokines, although the levels were lower when compared with responses of SA-14-14-2-immune splenocytes upon stimulation with JEV antigen. IL-2 and IFN-γ are predominant markers of the Th1 response; IL-4 expression is defined as a marker of the Th2 response. The results indicated that either the Th1- or the Th2-type cross-reactive immune response against ZIKV was evoked by the administration of the JEV SA-14-14-2 vaccine.
Cross-reactive cytokine responses in mouse splenocytes. a Schedule. Three weeks after the final immunization, splenocytes from adult C57BL/6 mice were collected. b The cytokines IL-2, IL-4, and IFN-γ secreted by splenocytes were determined using ELISPOT (n = 7). The numbers of cytokine-positive cells are reported as the mean SFU/3 × 105 splenocytes + SD. Asterisk indicates P < 0.05; double asterisk indicates P < 0.01; triple asterisk indicates P < 0.001
Cell-mediated immunity as a potential mechanism of cross-protection against ZIKV
Although multiple cytokines can be produced under ZIKV antigen stimulation, it was unclear whether cellular immunity was indispensable or essential for the in vivo cross-reactive protection. Therefore, adoptive transfer of immune splenocytes from SA14-14-2-immunized C57BL/6 mice into naïve Ifnar1-/- recipient mice was performed 3 weeks after the final immunization, followed by viral challenge with a lethal dose of JEV or ZIKV (Fig. 5a). After JEV challenge, mice receiving splenocytes derived from the control group showed marked and up to 26.7% body weight loss, and all mice died (0/6) within 9 days, whereas 100% (6/6) of mice receiving SA14-14-2-immune splenic lymphocytes survived without obvious weight change (Fig. 5b, c), suggesting that a protective prototype of splenocytes activated by SA14-14-2 was successfully established. Meanwhile, while naïve Ifnar1-/- recipient mice in the control group showed high susceptibility to ZIKV infection with weight loss of 17.0% and only 16.7% (1/6) survival rate, virtually 83.3% (5/6) of mice infused with SA14-14-2-immune lymphocytes survived the lethal ZIKV challenge with only a weight loss of 5.8% (Fig. 5b, c). Taken together with our nAb data, this result demonstrated that a cell-mediated cross-reactive response induced by SA14-14-2 immunization was protective against subsequent ZIKV infection.
Adoptive transfer of splenocytes from SA14-14-2-vaccinated C57BL/6 mice and cross-protection of splenocytes in Ifnar1-/- mice. a Schedule. Splenic lymphocytes (3 × 106 cells per mouse) collected 3 weeks post final immunization were adoptively transferred r.o. to naïve adult mice, and 1 day later mice were challenged with a lethal dose of either JEV or ZIKV. As a control, splenic lymphocytes from PBS-treated mice were transferred to naïve mice prior to challenge. Results were evaluated for b body weight change and c survival rate of mice 14 consecutive days post challenge (n = 6). Mice exhibiting more than 25% loss in weight were humanely euthanized for ethical reasons. Each experiment was independently repeated three times. Results are expressed as mean ± SD. Asterisk indicates P < 0.05; double asterisk P < 0.001
Discussion
It is becoming increasingly apparent that the pre-existing immunity triggered by primary flavivirus infection or vaccination can induce immunological cross-reactivity to secondary exposure with a genetically and antigenically closely related flavivirus. Immunological cross-reactions have been implicated in both protection and pathology, and there is some controversy on the consequences of the outcome of infection. An in vitro study showed an enhancement of ZIKV replication in the presence of DENV antibodies (Dejnirattisai et al. 2016). However, clinical cohort and case-control studies of individuals in dengue endemic regions suggest the opposite that pre-existing dengue immunity reduces the risk of symptomatic ZIKV infection and congenital ZIKV syndrome (Gordon et al. 2019; Pedroso et al. 2019; Rodriguez-Barraquer et al. 2019). Pantoja et al. studied the effects of pre-existing DENV immunity on ZIKV infection in vivo in rhesus macaques and confirmed that the previous exposure to DENV did not result in the enhancement of ZIKV pathogenesis (Pantoja et al. 2017). Intriguingly, there has been relatively little ZIKV infection reported in Asia, and it has been suggested that cross-reactive T cell responses generated by -borne flaviviruses, of which the principal agent is JEV, has limited ZIKV spread in Asia (Gaunt et al. 2019).
Previously, we found that live attenuated JE vaccine SA-14-14-2 conferred cross-reactive nAbs which contributed to the cross-protection against DENV challenge (Li et al. 2016). In contrast to this result, in this study, mice immunized with SA14-14-2 showed a cross-reactive IgG antibody response to ZIKV without the presence of neutralizing activity (Figs. 2b and 3b). Although the ADE in SA14-14-2 immune sera was detected in vitro (Fig. 3d), we found no evidence that this resulted in a harmful effect; indeed, in subsequent ZIKV challenge, SA14-14-2 vaccination slightly lengthened the median survival (Fig. 2d). This is an important result, suggesting that cross-reactive antibodies from SA14-14-2 vaccination are not pathogenic in vivo, because many people in Asia have received inactivated JE vaccine which will generate anti-JEV nAb but may not contain many of the cross-reactive T cell epitopes, which lie in the NS proteins (Turtle et al. 2016). Here, we use neonatal C57BL/6 mice instead of neonatal mice because the former is susceptible to ZIKV and can mimic the signs (Wang et al. 2018). Although the SA14-14-2 vaccine triggered both Th1 and Th2 responses (Figs. 4b and 3c), adoptive splenocyte transfer was superior to serum transfer in protection against ZIKV infection, implying a strong correlation between SA14-14-2-induced cellular immunity and the ZIKV cross-protective capacity. However, the limitation in this study is that we did not elucidate the functional components which are cross-protective by further sorting of splenocytes for adoptive transfer, such as purified T cells or their subpopulations (CD8+ or CD4+ alone), or cross-reactive memory B cells. Furthermore, we did not determine whether the cross-reactive nAb response was present in recipient mice transferred with SA14-14-2-immune splenocytes, although this is unlikely to change our primary conclusions.
Traditionally, based on cross-neutralizing activity, flaviviruses have been sub-divided into distinct serocomplexes. Cross-neutralization between different serocomplexes is usually not observed (Heinz and Stiasny 2017). The flavivirus E protein is the principal antigen against which the nAb response is directed. The extent of cross-neutralization correlates with the amino acid sequence identity of E protein: when the sequence identity in E protein is less than 40%, cross-neutralization is lost (Stiasny et al. 2006). JEV and ZIKV belong to distinct serocomplexes; although the homology of the E protein amino acid sequence between the JEV SA14-14-2 strain and the ZIKV SMGC-1 strain was 53.4%, the distinct epitopes in E proteins of JEV and ZIKV within different flaviviruses that dominate antibody responses are presumably responsible for the unavailable cross-neutralization (Dowd and Pierson 2011). For the sequence homology of the E protein, ZIKV is more closely related to the DENV than to the JEV serocomplex (Heinz and Stiasny 2017). However, most B cell epitopes are conformational (Sanchez-Trincado et al. 2017), and therefore, sequence homology may not fully reflect “relatedness” as measured by cross-reactive humoral responses. Interestingly, one structural model of the relatedness of flavivirus surface topology in fact placed ZIKV and JEV closer to each other than either was to DENV (Wang et al. 2017). Nevertheless, despite this structural similarity, this did not account for protection in the model we describe here.
In contrast, NS proteins among flaviviruses are more conserved with up to 68.0% identity than structural proteins. Weiskopf et al. indicated that, following heterologous DENV infection, memory CD8+ T cells expanded that recognized conserved NS proteins (Weiskopf et al. 2014). In fact, NS3 and NS5 represent the main targets of the CD8+ T cell response to flaviviruses (Dos Santos et al. 2019; Rivino and Lim 2017). One study reported more cross-reactive T cell responses to full-length NS3 helicase because of higher sequence homology than that to the protease region alone (Herrera et al. 2018). Also, a homologous analysis based on the NS5 protein would place ZIKV closer to the JEV serocomplex than to DENVs (Barba-Spaeth et al. 2016). In our study, we hypothesize that it is the cross-reactive response to shared T cell epitopes in the NS proteins that contributes to protection mediated by the adoptive transfer of immune splenocytes.
In a mouse model, CD8+ T cells can mediate protection against ZIKV (Dos Santos et al. 2019). DENV-specific cross-reactive CD8+ T cells can also protect (Wen et al. 2017), and similar responses are detected in humans, where cross-reactive CD8+ T cells specific for DENV display anti-ZIKV effector potential toward ZIKV, mediating direct cytolysis (Lim et al. 2018). JEV and ZIKV share 63.9% and 68.0% homology in NS3 and NS5, respectively. Our findings are consistent with a cross-reactive CD8+ T cell mediating protection, but characterization of the key components responsible for cross-protection in splenic lymphocytes and identification of JEV/ZIKV cross-reactive epitopes warrant further investigation.
Since 2007, China has included a two-shot schedule of the JEV SA14-14-2 vaccine into the national Expanded Program on Immunization. Children under the age of 13 and even older children in some provinces of China have pre-existing JEV immunity. It should be noted that, according to the established schedule, children received the SA14-14-2 vaccine at 8 months and 2 years of age, but three immunizations in mice were performed in this study, as per the earlier studies (Li et al. 2016). In order to ensure comparability with what humans receive, future studies may need to test various dosing regimens.
Fu et al. and Xiao et al. identified ZIKV in mosquitoes in Guizhou province and Yunnan province (Fu et al. 2017; Xiao et al. 2018), indicating that ZIKV is present in China. Therefore, it is inevitable that JEV and ZIKV co-circulate. The identification of ZIKV presents new challenges for prevention and control because of its severe consequences in pregnancy (Grazel and Harris-Haman 2018) and prompts an urgent need to clarify the mechanisms underlying such cross-protective immunity, which will inform the strategy of developing safe and effective vaccines targeting both viruses, with appropriately balanced B cell and T cell antigens.
A shortcoming of our work is that we have not performed more detailed mechanistic experiments to determine which components of the cellular response are responsible for mediating protection. A further hypothesis that remains to be explored is whether cellular immunity to the JE vaccine results in a faster ZIKV-specific nAb response, by providing T cell help to ZIKV specific B cells by cross-reactive CD4+ T cells. Detailed mapping and specific epitope cross-reactivity studies of the T cell response to JE vaccine SA14-14-2 in this model would address this. This, along with a more detailed definition of the mediators expressed by cross-reactive T cells, or other protective mechanisms, should be the priorities for ongoing work.
In conclusion, the results of our study demonstrated that JEV SA14-14-2 elicited effective cross-protection against ZIKV in mice. Our results indicate the potential for the widespread use of the vaccine, especially in those co-circulating countries. Moreover, this study will provide important information in terms of ZIKV prevention or precaution. Furthermore, it is worthwhile to identify common epitopes for the future development of a novel bivalent vaccine based on T cell against both JEV and ZIKV.
References
Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA (2016) Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536(7614):48–53. https://doi.org/10.1038/nature18938
Breitbach ME, Newman CM, Dudley DM, Stewart LM, Aliota MT, Koenig MR, Shepherd PM, Yamamoto K, Crooks CM, Young G, Semler MR, Weiler AM, Barry GL, Heimsath H, Mohr EL, Eichkoff J, Newton W, Peterson E, Schultz-Darken N, Permar SR, Dean H, Capuano S 3rd, Osorio JE, Friedrich TC, O'Connor DH (2019) Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLoS Pathog 15(8):e1007766. https://doi.org/10.1371/journal.ppat.1007766
Chang HH, Huber RG, Bond PJ, Grad YH, Camerini D, Maurer-Stroh S, Lipsitch M (2017) Systematic analysis of protein identity between Zika virus and other arthropod-borne viruses. Bull World Health Organ 95(7):517–525I. https://doi.org/10.2471/BLT.16.182105
Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR (2016) Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 17(9):1102–1108. https://doi.org/10.1038/ni.3515
Dos Santos FL, Gushi LT, Luiz WB, Amorim JH (2019) Seeking flavivirus cross-protective immunity. Front Immunol 10:2260. https://doi.org/10.3389/fimmu.2019.02260
Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R (2016) A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis 10(5):e0004658. https://doi.org/10.1371/journal.pntd.0004658
Dowd KA, Pierson TC (2011) Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology 411(2):306–315. https://doi.org/10.1016/j.virol.2010.12.020
Fowler AM, Tang WW, Young MP, Mamidi A, Viramontes KM, McCauley MD, Carlin AF, Schooley RT, Swanstrom J, Baric RS, Govero J, Diamond MS, Shresta S (2018) Maternally acquired Zika antibodies enhance dengue disease severity in mice. Cell Host Microbe 24(5):743–750 e5. https://doi.org/10.1016/j.chom.2018.09.015
Fu S, Song S, Liu H, Li Y, Li X, Gao X, Xu Z, Liu G, Wang D, Tian Z, Zhou J, He Y, Lei W, Wang H, Wang B, Lu X, Liang G (2017) ZIKA virus isolated from mosquitoes: a field and laboratory investigation in China, 2016. Sci China Life Sci 60(12):1364–1371. https://doi.org/10.1007/s11427-017-9196-8
Gao N, Li J, Sheng Z, Chen H, Fan D, Wang P, An J (2019) Japanese encephalitis virus prM-E antigen immunization conferred protection against challenge by four different serotypes of Dengue viruses in mice. Appl Microbiol Biotechnol 103(12):4977–4986. https://doi.org/10.1007/s00253-019-09798-9
Gaunt MW, Gubler DJ, Pettersson JH, Kuno G, Wilder-Smith A, de Lamballerie X, Gould EA, Falconar AK (2019) Recombination of B- and T-cell epitope-rich loci from Aedes- and Culex-borne flaviviruses shapes Zika virus epidemiology. Antivir Res 174:104676. https://doi.org/10.1016/j.antiviral.2019.104676
Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kolsch E, Podlaski FJ, Gately MK, Rude E (1995) Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol 25(3):823–829. https://doi.org/10.1002/eji.1830250329
Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez N, Mercado JC, Chowell G, Lopez B, Elizondo D, Coloma J, Burger-Calderon R, Kuan G, Balmaseda A, Harris E (2019) Prior dengue virus infection and risk of Zika: a pediatric cohort in Nicaragua. PLoS Med 16(1):e1002726. https://doi.org/10.1371/journal.pmed.1002726
Grazel R, Harris-Haman P (2018) Zika virus infection: a vector-borne threat to pregnant women and infants. Adv Neonatal Care 18(5):350–359. https://doi.org/10.1097/ANC.0000000000000557
Grossman RA, Edelman R, Gould DJ (1974) Study of Japanese encephalitis virus in Chiangmia Valley, Thailand. VI. Summary and conclusions. Am J Epidemiol 100(1):69–76. https://doi.org/10.1093/oxfordjournals.aje.a112010
Guarner J, Hale GL (2019) Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: West Nile, Zika, dengue and yellow fever. Semin Diagn Pathol 36(3):170–176. https://doi.org/10.1053/j.semdp.2019.04.009
Heinz FX, Stiasny K (2017) The antigenic structure of Zika virus and its relation to other flaviviruses: implications for infection and immunoprophylaxis. Microbiol Mol Biol Rev 81(1). https://doi.org/10.1128/MMBR.00055-16
Herrera BB, Tsai WY, Chang CA, Hamel DJ, Wang WK, Lu Y, Mboup S, Kanki PJ (2018) Sustained specific and cross-reactive T cell responses to Zika and dengue virus NS3 in West Africa. J Virol 92(7). https://doi.org/10.1128/JVI.01992-17
Langerak T, Mumtaz N, Tolk VI, van Gorp ECM, Martina BE, Rockx B, Koopmans MPG (2019) The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLoS Pathog 15(4):e1007640. https://doi.org/10.1371/journal.ppat.1007640
Lecouturier V, Bernard MC, Berry C, Carayol S, Richier E, Boudet F, Heinrichs J (2019) Immunogenicity and protection conferred by an optimized purified inactivated Zika vaccine in mice. Vaccine 37(20):2679–2686. https://doi.org/10.1016/j.vaccine.2019.04.013
Li J, Gao N, Fan D, Chen H, Sheng Z, Fu S, Liang G, An J (2016) Cross-protection induced by Japanese encephalitis vaccines against different genotypes of Dengue viruses in mice. Sci Rep 6:19953. https://doi.org/10.1038/srep19953
Lim MQ, Kumaran EAP, Tan HC, Lye DC, Leo YS, Ooi EE, MacAry PA, Bertoletti A, Rivino L (2018) Cross-reactivity and anti-viral function of dengue capsid and NS3-specific memory T cells toward Zika virus. Front Immunol 9:2225. https://doi.org/10.3389/fimmu.2018.02225
Mendez N, Oviedo-Pastrana M, Mattar S, Caicedo-Castro I, Arrieta G (2017) Zika virus disease, microcephaly and Guillain-Barre syndrome in Colombia: epidemiological situation during 21 months of the Zika virus outbreak, 2015-2017. Arch Public Health 75:65. https://doi.org/10.1186/s13690-017-0233-5
Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, Martinez MI, Hassert MA, Brien JD, Pinto AK, de Silva A, Sariol CA (2017) Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 8:15674. https://doi.org/10.1038/ncomms15674
Pedroso C, Fischer C, Feldmann M, Sarno M, Luz E, Moreira-Soto A, Cabral R, Netto EM, Brites C, Kummerer BM, Drexler JF (2019) Cross-protection of dengue virus infection against congenital Zika syndrome, Northeastern Brazil. Emerg Infect Dis 25(8):1485–1493. https://doi.org/10.3201/eid2508.190113
Rivino L, Lim MQ (2017) CD4(+) and CD8(+) T-cell immunity to dengue - lessons for the study of Zika virus. Immunology 150(2):146–154. https://doi.org/10.1111/imm.12681
Rodriguez-Barraquer I, Costa F, Nascimento EJM, Nery NJ, Castanha PMS, Sacramento GA, Cruz J, Carvalho M, De Olivera D, Hagan JE, Adhikarla H, Wunder EA Jr, Coelho DF, Azar SR, Rossi SL, Vasilakis N, Weaver SC, Ribeiro GS, Balmaseda A, Harris E, Nogueira ML, Reis MG, Marques ETA, Cummings DAT, Ko AI (2019) Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 363(6427):607–610. https://doi.org/10.1126/science.aav6618
Sanchez-Trincado JL, Gomez-Perosanz M, Reche PA (2017) Fundamentals and methods for T- and B-cell epitope prediction. J Immunol Res 2017:2680160–2680114. https://doi.org/10.1155/2017/2680160
Shan C, Xie X, Luo H, Muruato AE, Liu Y, Wakamiya M, La JH, Chung JM, Weaver SC, Wang T, Shi PY (2019) Maternal vaccination and protective immunity against Zika virus vertical transmission. Nat Commun 10(1):5677. https://doi.org/10.1038/s41467-019-13589-1
Slon-Campos JL, Dejnirattisai W, Jagger BW, Lopez-Camacho C, Wongwiwat W, Durnell LA, Winkler ES, Chen RE, Reyes-Sandoval A, Rey FA, Diamond MS, Mongkolsapaya J, Screaton GR (2019) A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nat Immunol 20(10):1291–1298. https://doi.org/10.1038/s41590-019-0477-z
Stiasny K, Kiermayr S, Holzmann H, Heinz FX (2006) Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol 80(19):9557–9568. https://doi.org/10.1128/JVI.00080-06
Strauss JH, Strauss EG (2001) Virus evolution: how does an enveloped virus make a regular structure? Cell 105(1):5–8. https://doi.org/10.1016/s0092-8674(01)00291-4
Turtle L, Bali T, Buxton G, Chib S, Chan S, Soni M, Hussain M, Isenman H, Fadnis P, Venkataswamy MM, Satishkumar V, Lewthwaite P, Kurioka A, Krishna S, Shankar MV, Ahmed R, Begum A, Ravi V, Desai A, Yoksan S, Fernandez S, Willberg CB, Kloverpris HN, Conlon C, Klenerman P, Satchidanandam V, Solomon T (2016) Human T cell responses to Japanese encephalitis virus in health and disease. J Exp Med 213(7):1331–1352. https://doi.org/10.1084/jem.20151517
Wang X, Li SH, Zhu L, Nian QG, Yuan S, Gao Q, Hu Z, Ye Q, Li XF, Xie DY, Shaw N, Wang J, Walter TS, Huiskonen JT, Fry EE, Qin CF, Stuart DI, Rao Z (2017) Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat Commun 8(1):14. https://doi.org/10.1038/s41467-017-00024-6
Wang R, Liao X, Fan D, Wang L, Song J, Feng K, Li M, Wang P, Chen H, An J (2018) Maternal immunization with a DNA vaccine candidate elicits specific passive protection against post-natal Zika virus infection in immunocompetent BALB/c mice. Vaccine 36(24):3522–3532. https://doi.org/10.1016/j.vaccine.2018.04.051
Wang R, Gao N, Li Y, Fan D, Zhen Z, Feng K, Chen H, An J (2019a) Cross-protection against four serotypes of dengue virus in mice conferred by a Zika DNA vaccine. Front Cell Infect Microbiol 9:147. https://doi.org/10.3389/fcimb.2019.00147
Wang R, Xie L, Gao N, Fan D, Chen H, Wang P, Zhou H, An J (2019b) Decreases in both the seroprevalence of serum antibodies and seroprotection against Japanese encephalitis virus among vaccinated children. Virol Sin 34(3):243–252. https://doi.org/10.1007/s12250-019-00099-z
Wang R, Zheng X, Sun J, Feng K, Gao N, Fan D, Chen H, Jin X, An J (2019c) Vaccination with a single consensus envelope protein ectodomain sequence administered in a heterologous regimen induces tetravalent immune responses and protection against dengue viruses in mice. Front Microbiol 10:1113. https://doi.org/10.3389/fmicb.2019.01113
Wang R, Yang FJ, Zheng XY, Liao XZ, Fan DY, Chen H, An J (2020) Long-term protection against dengue viruses in mice conferred by a tetravalent DNA vaccine candidate. Zool Res 41(1):90–93. https://doi.org/10.24272/j.issn.2095-8137.2020.016
Weiskopf D, Angelo MA, Sidney J, Peters B, Shresta S, Sette A (2014) Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection. J Virol 88(19):11383–11394. https://doi.org/10.1128/JVI.01108-14
Wen J, Elong Ngono A, Regla-Nava JA, Kim K, Gorman MJ, Diamond MS, Shresta S (2017) Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat Commun 8(1):1459. https://doi.org/10.1038/s41467-017-01669-z
Xiao P, Han J, Zhang Y, Li C, Guo X, Wen S, Tian M, Li Y, Wang M, Liu H, Ren J, Zhou H, Lu H, Jin N (2018) Metagenomic analysis of Flaviviridae in mosquito viromes isolated from Yunnan Province in China reveals genes from dengue and Zika viruses. Front Cell Infect Microbiol 8:359. https://doi.org/10.3389/fcimb.2018.00359
Zhou J, Chi X, Cheng M, Huang X, Liu X, Fan J, Xu H, Lin T, Shi L, Qin C, Yang W (2019) Zika virus degrades the omega-3 fatty acid transporter Mfsd2a in brain microvascular endothelial cells and impairs lipid homeostasis. Sci Adv 5(10):eaax7142. https://doi.org/10.1126/sciadv.aax7142
Zimmerman MG, Quicke KM, O'Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, Johnson EL, Cordes S, Haddad L, Chakraborty R, Coyne CB, Wrammert J, Suthar MS (2018) Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe 24(5):731–742 e6. https://doi.org/10.1016/j.chom.2018.10.008
Funding
This study was supported by the National Natural Science Foundation of China (grant numbers 81772172 to Hui Chen and 81671971 to Jing An), and the Cultivation Fund Project of the National Natural Science Foundation in Beijing Children’s Hospital, Capital Medical University (grant number GPQN201909 to Ran Wang), which funded the experimental work. Lance Turtle is supported by the Wellcome Trust (grant number 205228/Z/16/Z) and EU Horizon 2020 ZikaPLAN (Preparedness Latin America Network) consortium (grant number 734584).
Author information
Authors and Affiliations
Contributions
Ran Wang designed and performed experiments, analyzed data, and wrote the manuscript. Zida Zhen, Baohua Hou, and Yueqi Li helped to perform experiments. Lance Turtle interpreted data and assisted in writing the manuscript. Na Wu, Na Gao, and Dongying Fan provided valuable suggestions for manuscripts and experiments. Hui Chen designed the experiment and reviewed the manuscript. Jing An reviewed and guided the overall experiment. All authors have critically read and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. The animal experiments were performed in strict accordance with the recommendations in the national guidelines for the use of animals in scientific research “Regulations for the Administration of Affairs Concerning Experimental Animals.” All experimental procedures were approved by the Institutional Animal Care and Use Committee of Capital Medical University, China. All efforts were made to minimize suffering.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Zhen, Z., Turtle, L. et al. T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine. Appl Microbiol Biotechnol 104, 6779–6789 (2020). https://doi.org/10.1007/s00253-020-10710-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10710-z