Abstract
Nitrate has been reported to improve tolerance of plants under flooding by modulating carbon and nitrogen metabolism of root cells; however, the extent to which nitrate modulates the photosynthetic process is not well understood. This work aimed to evaluate the photosynthetic process by nitrate-mediated modulation of hypoxic metabolism in the root of common bean plants (Phaseolus vulgaris L.) under flooding and recovery conditions. Three groups of common beans [N2-fixing (N2)], N2-fixing pre-hypoxic nitrate-treated (N2 + NO3−), and nitrate (NO3−)-supplied plants] were grown in vermiculite and the root system was subjected to flooding for 48 h at the early reproductive stage. After flooding, plants returned to normoxic conditions per 24 and 72 h for recovery. Plants from N2 + NO3− and mainly NO3− groups maintained transpiration rate (E) and CO2 assimilation under root-flooding, which was not shown by N2-fixing plants. No changes in the dynamic dissipation of photosynthetic energy were evidenced in leaves of NO3− plants upon flooding, besides an increase in nitrate reductase (NR) activity and a decrease in fermentative enzymes in roots. Reactive oxygen species (ROS) and antioxidative enzymes increased in leaves and decreased in roots. Nitrate-mediated maintenance of the photosynthetic process may be related to induction in NR activity in roots to alleviate the toxic effects of fermentation through a decrease of fermentative enzyme activity.
Similar content being viewed by others
Introduction
Common bean (Phaseolus vulgaris L.) is an important food in Brazil and can provide up to 30% of protein for human consumption. However, about 28 million hectares are flood-prone located in the lowlands of Cerrado and in the South of Brazil (Jackson and Colmer 2005). Flooded areas are increasing worldwide due to climate changes (Bailey-Serres et al. 2012; Limami et al. 2014) which reduces food production (USDA 2015). Such areas can be used to increase food production in a crop rotation with the current rice cropping system, besides that, the use of legume plants in a crop rotation can improve soil conditions especially by providing nitrogen, break pests and diseases and contributing to reducing weed cycles (Garcia et al. 2020).
Legumes, such as common bean (Phaseolus vulgaris L.), are sensitive to flooding conditions (Loureiro et al. 1998; Amarante et al. 2006; Sánchez et al. 2011; Justino and Sodek 2013; Borella et al. 2017; Posso et al. 2018). Flooded soils limit the oxygen diffusion in water which is 104 times slower than in air (Armstrong et al. 1994). Flooding impairs oxygen supply to the roots and nodules, shifting aerobic to anaerobic respiration (Limami et al. 2014). Hypoxia increases the "Pasteur effect" in root cells by speeding up glycolytic reactions to provide energy supply due to inhibition in oxidative phosphorylation (Summers et al. 2000). To keep glycolysis running in root cells under flooding, the fermentative pathway is rapidly activated to continuously regenerate NAD+ and maintain low ATP production at level substrate (van Dongen and Licausi 2015).
Several studies have reported that exogenous nitrate supplementation to plants increases the redox state in root tissues upon hypoxia conditions (Stoimenova et al. 2003; Oliveira et al. 2013b; van Dongen and Licausi 2015). The cytosolic NR acts in an alternative way of recycling NAD+ from NADH, through the conversion of nitrite to nitric oxide (NO) (Oliveira et al. 2013b; Limami et al. 2014; van Dongen and Licausi 2015). NO is reduced back to nitrate by a class of non-symbiotic hemoglobins (nsHb) (Pucciariello and Perata 2017), known as a NO cycle (van Dongen and Licausi 2015). The functioning of the NO cycle contributes for the recycling of NAD+ and reduces the effects of the main end-products of fermentation (van Dongen and Licausi 2015), promoted by ethanol and lactate production via alcohol dehydrogenase (ADH) and lactate dehydrogenase (LDH), respectively (Zabalza et al. 2009; Pucciariello and Perata 2017).
Hypoxia causes cytosol acidification which leads to aquaporin inhibition and consequently, limits water uptake by roots and hydraulic conductivity through the xylem sap. In shoots, the result of reduced water transport from roots may lead to stomata closure, diminishing E and stomatal conductance (gs) (Tournaire-Roux et al. 2003; Aroca et al. 2012; Limami et al. 2014). As nitrate supplementation alleviates the effects of hypoxia in root cells (Stoimenova et al. 2003; Oliveira et al. 2013b; van Dongen and Licausi 2015; Borella et al. 2017), we hypothesized that the positive involvement of nitrate in root cells could be extended to shoot by maintaining E and gs to maintain CO2 assimilation, reported to decrease under flooding conditions (Ahmed et al. 2002; Bansal and Srivastava 2015; Posso et al. 2018). Besides, plants maintain the dynamic dissipation of the photosynthetic energy of common bean plants (Posso et al. 2018) and avoid photo-oxidative damages (Bräutigam and Gowik 2016; Hanawa et al. 2017).
Conditions of hypoxia and especially cell re-oxygenation upon return of the normoxic conditions lead to ROS production (Murphy 2009), such as superoxide (O2•−) and hydrogen peroxide (H2O2) (Ivanov et al. 2018), promoted by an imbalance in photosynthetic ETC in common bean plants (Posso et al. 2018). Besides the signaling mechanisms, excessive ROS production can trigger oxidative damages to membrane lipids (causing lipid peroxidation), protein, DNA, RNA and nucleosides (Gill and Tuteja 2010; Ivanov et al. 2018). Injuries can be avoided by the ability of root and shoot cells to cope with ROS production via induction of the antioxidative defence system composed of enzymes (such as superoxide dismutase, catalase, ascorbate peroxidase) and non-enzymatic low molecular weight compounds (Blokhina and Fagerstedt 2010a, b).
The beneficial effects of nitrate supplementation in the root of plants under flooding conditions has been extensively studied regarding carbon and nitrogen metabolism (Patterson et al. 2010; Oliveira et al. 2013a, b). However, the extent effects of nitrate in the shoot of plants with roots under hypoxia remain to be elucidated, especially in common bean plants, where it may play a beneficial role in the maintenance of photosynthetic metabolism in short periods of flooding. Thus, the aim of this work was to evaluate the photosynthetic process by nitrate-mediated modulation of hypoxic metabolism in root cells of common bean plants under flooding and recovery conditions.
Materials and methods
Plant material and growth conditions
Common bean seeds (Phaseolus vulgaris L. cv. BRS Expedito) were sown with and without inoculation in a polyethylene tray (20 L) containing vermiculite as substrate and kept in a greenhouse. The seeds were inoculated with Rhizobium tropicii strain SEMIA 4080 (inoculum Nitrafix beans—Stoller®, according to the manufacturer’s recommendation). When the seedlings reached the V1 stage (Osorno et al. 2014), one plant was transplanted per 1.5-L pot containing fine vermiculite as substrate. The plants were supplied with 100 mL of nutrient solution (Hoagland and Arnon 1938), containing nitrogen in the form of potassium nitrate (KNO3) for plants without inoculation (non-nodulated) and N-free nutrient solution for inoculated plants, three times per week and watered daily. N-fixing in N-free plants started at early vegetative growth stage (V3) when leaves turned fully green. The flooding treatments were applied at the reproductive stage (R1; Osorno et al. 2014), to avoid N-starvation. The whole experiment was carried out in a greenhouse under natural light (average photosynthetic active radiation during the experiment ranged from 660 to 1.034 μmol photons m−2 s−1) and temperature conditions of 25 ± 5 °C.
At the reproductive stage (R1; Osorno et al. 2014), the plants were subjected to flooding of the root system by applying nutrient solution up to 2 cm above the substrate surface, applied according to the group of plants. The first one was grown as nodulated plants and the flooding treatment was with N-free nutrient solution (N2—symbiotic fixation with nitrogen available in ammonium form); the second group was grown as nodulated one and the flooding treatment was with nutrient solution containing 5 mM KNO3 [being pre-treated with 50 mL (5 mM) of nutrient solution containing nitrate at 5 and 3 days before flooding treatment], and named as N2 + NO3−; the third group was grown as non-nodulated plants (supplied with KNO3) and the flooding treatment was with nutrient solution containing 5 mM KNO3 (named NO3−). The nutrient solution applied during the flooding treatment was one-third of normal strength in all the three groups of plants. The nutrient solution was applied to maintain the nutrient uptake by plants during flooding conditions (Oliveira et al. 2013c). The whole root system was kept flooded for 48 h and afterward, it was drained for recovery per 24 and 72 h (post-hypoxia). Plants were kept under normoxia as a control treatment. At harvest, four biological replicates of roots and/or leaves were collected for each treatment and kept frozen (− 80 ºC) to perform biochemical analysis. Non-destructive analyses were performed as well as indicated below.
Measurements of Chl a fluorescence transients
The Handy-Pea fluorimeter (Plant efficiency analyser, Hansatech Instruments Ltd, UK) was used to access the fast Chl a fluorescence transients in intact leaves (first fully expanded trifoliate; non-destructive analysis) still attached to the plant and dark-adapted in a specially provided clip for at least 30 min before the measurements. The Chl a fluorescence was induced by one saturating red-light flash (peak at 650 nm) with 3.000 μmol photons m−2 s−1. The measurements were taken from the lowest intensity (FO—minimum fluorescence) to the highest intensity (FM—maximum fluorescence) during 1 s of illumination (Strasser et al. 2004). The intensity measured at 50 μs was considered as the initial fluorescence (F0) and considered the O-step (50 µs), followed by the intermediate levels [J- (2 ms) and I-steps (30 ms)] and the peak level [P-step (500 ms–1 s)], analyzed using the JIP-test parameters (for analysis of Chl a fluorescence data see Strasser et al. (2004) and Tsimilli-Michael and Strasser (2008). The OJIP transients were normalized as relative variable fluorescence: Wt = (Ft − F0)/(FJ − F0), WOI = (Ft − F0)/(FI − F0) and WIP = (Ft − FI)/(FP − FI), show the events in the OJ-, OI-, and IP-phases. In addition, the hidden bands between the steps WOJ (K-band; 300 µs) and WOK (L-band; 150 µs) were also calculated (ΔW = Wflooded or recovery − Wcontrol) as difference kinetics from relative variable fluorescence (Yusuf et al. 2010). The plotted fluorescence values were the average of 15 measurements taken from each treatment.
Gas exchange analysis
The portable infra-red CO2 analyser (model LI-6400XT LI-COR, Inc., Lincoln, NE, USA), with an in-chamber CO2 concentration of 380 µmol mol−1 and a photon flow density of 1.250 µmol photons m−2 s−1, provided by a light source (LI-COR 6400-02B) attached to the measuring chamber (2 × 3 cm) at 25 ºC (block temperature control) was used for gas exchange measurements in intact leaves (first fully expanded trifoliate; non-destructive analysis) still attached to the plant. The measurements were taken between 10:00 and 11:00 AM., and the data acquired followed the calculations described by von Caemmerer and Farquhar (1981).
ROS and lipid peroxidation measurements
The generation rate of O2·− was determined according to Elstner and Heupel (1976) and following the modifications of Li et al. (2010). Leaf and root samples (± 0.2 g) were powdered in liquid nitrogen and homogenized in 65 mM potassium phosphate buffer (pH 7.8). The homogenate was centrifuged at 5,000 g at 4 °C for 10 min. The supernatant was mixed with 65 mM potassium phosphate buffer (pH 7.8), 10 mM hydroxylamine and incubated at 25 °C for 20 min. Subsequently, 17 mM sulfanilamide and 7 mM α-naphthylamine were added in the reaction and incubated for 20 min at 25 °C, afterward, the absorbance of the solution was recorded at 530 nm. To calculate the generated rate of O2·−, a standard curve was performed with known concentrations of nitrogen dioxide (NO2). The H2O2 and lipid peroxidation levels were determined in leaves and roots (± 0.2 g). The tissues were powdered using a mortar and pestle with liquid nitrogen and homogenized with 0.1% (w: v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000 g at 4 ºC for 20 min. The supernatant was used to determine the H2O2 content according to Velikova et al. (2000) and lipid peroxidation as described by Cakmak and Horst (1991). Lipid peroxidation was determined using thiobarbituric acid (TBA), which determines malondialdehyde (MDA) as an end-product of lipid peroxidation. The molar extinction coefficient (ε = 155 × 103 M−1 cm−1) was used to calculate the amount of MDA–TBA complex (red pigment). The data were submitted to log10 = (flooded or recovery/control).
Glycolate oxidase activity
Glycolate oxidase enzyme activity (GO; EC 1.1.3.1) was determined in leaf tissue (± 0.2 g). Plant material was powdered in liquid nitrogen using a mortar and pestle and homogenized with 5% polyvinylpolypyrrolidone (PVPP) and 50 mM Tris–HCl buffer (pH 7.8) containing 0.01% Triton X-100 and 5 mM dithiothreitol (DTT). The homogenate was centrifuged (12,000 g, 20 min, 4 ºC). From the supernatant, an aliquot was added in a medium reaction containing 50 mM Tris–HCl (pH 7.8), 0.009% Triton X-100 (v/v), 3.3 mM phenylhydrazone HCl (pH 6.8) and 5 mM glycolate (pH 7.0), as described by Bai et al. (2014). GO activity was assayed by following the formation of glyoxylate phenylhydrazone at 30 ºC and the absorbance at 324 nm recorded at 10-s intervals. The data were submitted to log10 = (flooded or recovery/control), and the results plotted as a heatmap.
Antioxidative enzyme activity
To determine antioxidative enzyme activity, leaves and roots (± 0.2 g) were powdered in liquid nitrogen using a mortar and pestle and homogenized with 5% (w: v) PVPP and 100 mM potassium phosphate buffer, pH 7.8, containing 0.1 mM ethylenediaminetetraacetic acid (EDTA) and 20 mM sodium ascorbate. All procedures were carried out at 4 ºC. The homogenate was centrifuged (12,000 g, 20 min), and an aliquot of the supernatant was used as a crude enzyme extract in a medium reaction described by Azevedo Neto et al. (2006). Superoxide dismutase (SOD; EC 1.15.1.1) activity was assayed by monitoring the inhibition of the nitro blue-tetrazolium (NBT) coloration at 560 nm. Catalase (CAT; EC 1.11.1.6) activity was assayed by monitoring the decline in the absorbance at 240 nm. Ascorbate peroxidase (APX; EC 1.11.1.11) activity was assayed through ascorbate oxidation at 290 nm. Guaiacol peroxidase (GPOD; EC 1.11.1.7) activity was assayed by monitoring the tetraguaiacol production at 470 nm. The reaction medium was incubated at 30 ºC and the absorbance recorded at 10-s intervals. The data were submitted to log10 = (flooded or recovery/control), and the results plotted as a heatmap.
Fermentative enzyme activity
For the measurement of fermentative enzymes, root tissues (± 0.2 g) were ground to a powder using a mortar and pestle with liquid nitrogen and homogenized with 5% (w:v) PVPP and extraction buffer (50 mM Tris–HCl, pH 7.5, containing 1 mM DTT). The homogenate was centrifuged at 12,000 g for 20 min at 4 ºC. The supernatant was desalted using a PD-10 column (GE Healthcare, Buckinghamshire, UK). The eluted protein fraction was specifically assayed for ADH (EC 1.1.1.1) and LDH (EC 1.1.1.17). The total protein content of the enzyme extract was measured as described by Bradford (1976). For ADH and LDH activities, the enzymatic assays were performed spectrophotometrically by monitoring the oxidation of NADH at 340 nm recorded at 5-s intervals, during 3 min at 30 ºC. The ADH assay was monitored in acetaldehyde → ethanol direction in a reaction mix containing 50 mM K-phosphate buffer (pH 7.0), 0.6 mM NADH and 5.0 mM acetaldehyde to initiate the reaction (Hanson et al. 1984). The LDH assay was monitored in pyruvate → lactate direction in a reaction mix containing 50 mM Tris–HCl buffer (pH 7.5), 0.6 mM NADH, 3.0 µM potassium cyanide, 0.2 mM 4-methylpyrazole and 10 mM sodium-pyruvate to initiate the reaction (Hanson and Jacobsen 1984).
The in vivo NR activity
The in vivo method was used to determine the activity of NR (EC 1.6.6.1), in fresh segments of leaves and roots. Fresh tissues were vacuum infiltrated with a vacuum pump (applying a pressure of − 50 kPa during 1 min for five times, intercalated with a period of 30 s of resting in each time, maintaining the pressure in the desiccator) in a reaction medium containing 50 mM potassium phosphate buffer, pH 7.5, 50 mM KNO3 and 1% propanol. After that, the samples were incubated at 30 °C in the dark (Jaworski 1971). Aliquots were removed at 0 and 30 min for the determination of nitrite (NO2−), followed by the method of Hageman and Reed (1980).
Statistical analysis
The experiment was carried out in a fully randomized design, consisting of one plant per pot (experimental unit) and five replicates per treatment. The data of gas exchange, glycolate oxidase, antioxidative and fermentative enzymes, ROS, lipid peroxidation, and NR activity were submitted to analysis of variance (ANOVA). When F was significant, the means of flooded or recovery were compared to the control by the t test (P ≤ 0.05) and among the groups of plants (N2, N2 + NO3− and NO3−) by the Tukey test (P ≤ 0.05), both in each period of harvesting. Statistical analyses were performed using SAS 9.0 statistical software program (SAS Institute Inc. Cary, NC, USA).
Results
Chl a fluorescence of plants under flooding conditions
The effects of root flooding on Chl a fluorescence transients of common bean plants, measured from 50 µs up to 1 s in dark-adapted leaves are shown in Figs. 1, 2 and 3. Common bean plants from all treatments showed a typical polyphasic Chl a fluorescence (Wt) transients (with OJIP-steps on logarithmic timescale marked in the plot; Fig. 1a, b, c). Although a sigmoid curve was showed by plants in all treatments, N2-fixing plants subjected to 48 h of flooding showed a marked increase in the J- and I-steps (2 and 30 ms, respectively), compared to plants under normoxia. When N2-fixing plants were pre-hypoxic treated with nitrate (N2 + NO3−) the fluorescence was slightly increased, and plants only supplied with nitrate (NO3−) did not show alterations in the polyphasic fluorescence curves, both in comparison to its respective control upon 48 h of flooding (Fig. 1a). The return to normoxic conditions did not decrease the fluorescence induction in N2-fixing plants, even with 72 h of recovery, whereas the fluorescence in N2 + NO3− plants was similar to the control with 24 h of recovery (Fig. 1c, d).
Chl a fluorescence transients (OJIP) of dark-adapted leaves of common bean plants subjected to flooding (a and d) and recovery (b, c, e, and f) conditions and different nitrogen sources. a–c Relative variable fluorescence between the steps O and P (Wt) on logarithmic time. d–f Photosynthetic parameters deduced by JIP-test analysis of fluorescence transients normalized using as reference the control. NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants. ms = milliseconds. (n = 15)
Chl a fluorescence transients (OJIP) of dark-adapted leaves of common bean plants subjected to flooding (a and d) and recovery (b, c, e, and f) conditions and different nitrogen sources. a–c Relative variable fluorescence between the steps O and I (WOI) on logarithmic time scale. d–f Relative variable fluorescence between the steps I and P (WIP) and WOI in the insert. NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants. ms = milliseconds. (n = 15)
Chl a fluorescence transients kinetics (OJIP) of dark-adapted leaves of common bean plants subjected to flooding (a and d) and recovery (b, c, e, and f) conditions and different nitrogen sources. a–c Average kinetics between the steps O and K (ΔWOK). d–f Average kinetics between the steps O and J (ΔWOJ). NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants. ms = milliseconds. (n = 15)
Functional and structural parameters (analysed by the JIP-test, see Strasser and Strasser 1995; Strasser et al. 2004) are depicted in Fig. 1d, e, f to show the photosynthetic behavior of plants assimilating different sources of nitrogen under root flooding conditions. The energy dissipation as heat per reaction center (DI0/RC) increased substantially in N2-fixing and N2 + NO3− plants with 48 h of flooding while did not affect NO3−-supplied plants. On the other hand, flooding reduced the functionality of ETC, as evidenced by a reduction in PIABS [performance index (potential) for energy conservation from exciton to the reduction of intersystem electron acceptors] and PItotal [performance index (potential) for energy conservation from exciton to the reduction of photosystem I (PSI) end acceptors].
Negative effects of flooding are extended by N2-fixing plants upon recovery (24 and 72 h). Under these conditions, N2-fixing plants increased the energy fluxes for (light) absorption (ABS/RC), trapping (TR0/RC) and heat (DI0/RC), while nitrate-supplied plants (N2 + NO3− and NO3−) did not show alterations in these parameters. Similarly, N2-fixing plants also decreased quantum yields and efficiencies (Fig. 1e, f), such as ψEo [efficiency/probability that an electron moves further than quinone A (QA−)], φEo [quantum yield for electron transport (ET)], φRo [quantum yield for reduction of end electron acceptors at the PSI acceptor side (RE)], δRo (efficiency/probability with which an electron from the intersystem electron carriers moves to reduce end electron acceptors at the PSI acceptor side) and performance indexes (PIABS and PItotal).
Besides alterations in parameters of the JIP-test, the normalization of Chl a fluorescence between the steps O and I showed an increase in the sequence of events from exciton trapping by photosystem II (PSII) up to plastoquinone (PQ) reduction (WOI; Fig. 2), with higher increase in N2-fixing plants upon root flooding (Fig. 2a) and recovery (Fig. 2b, c), while N2-fixing plants supplied with nitrate (N2 + NO3−), showed decrease only with 48 h of flooding and nitrate-supplied plants (NO3−), did not show no alterations compared to control. In addition, N2-fixing plants decreased the electron transfer starting at PQH2 (plastoquinol) to the end electron acceptors on the PSI acceptor side (WIP and WOI > 1 in the insert; Fig. 2d–f) and did not recover even with 72 h after normoxic re-establishment. On the other hand, the electron transfer at this point remained similar to control in N2 + NO3− plants and especially in nitrate-supplied plants (NO3−).
Additional normalizations and corresponding subtractions revealed the presence of both hidden L- [between the steps O and K (ΔWOK); Fig. 3a–c] and K-bands [between the steps O and J (ΔWOJ); Fig. 3d–f]. Positive L-band in N2-fixing plants (48 h of flooding and 24 and 72 h of recovery) and N2 + NO3− plants (48 h of flooding) showed lower energetic connectivity between PSII and PSI, inefficient consumption of the excitation energy and low stability of the system (Yusuf et al. 2010). Positive K-band in N2-fixing and N2 + NO3− plants under flooding and recovery, with stronger effects in N2-fixing plants upon recovery (Fig. 3d–f), reflected an inactivation of the oxygen-evolving complex (OEC), which was not evidenced in NO3− plants. It is noteworthy to emphasize that nitrate-supplied plants (NO3−) did not show alterations in the dynamic dissipation of the photosynthetic energy upon flooding and recovery conditions, while N2-fixing plants showed pronounced positive effects upon nitrate supplementation (Figs. 1, 2 and 3).
Gas exchange under root flooding conditions
Root flooding (48 h) induced a decrease in net assimilation rate (40%; Fig. 4a) and E (43%; Fig. 4g), only in N2-fixing plants. The decrease in these parameters was extended up to 24 h of recovery (50%—net assimilation rate; 63%—E), being re-established upon 72 h of recovery. Plants supplied with nitrate (N2 + NO3− and NO3−) did not decrease the CO2 assimilation under flooding and recovery conditions, maintaining the photosynthetic process compared to N2-fixing plants. The reduction in CO2 assimilation was the result of an expressive reduction in gs (Fig. 4d–f), which was strongly reduced in about threefold with 48 h of flooding (Fig. 4d) and twofold with 24 h of recovery (Fig. 4e) in N2-fixing plants in comparison to control one and in comparison to plants supplied with nitrate under flooding and recovery conditions. Nitrate-supplied plants did not show alterations in gas exchange upon flooding and recovery conditions.

source of nitrogen. Means followed by the same letters do not differ by Tukey’s test (P ≤ 0.05) among treatments (lowercase letter for control groups and capital letter for treated one). NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants
Gas Exchange in leaves of common bean plants subjected to flooding (a, d and g) and recovery (b, c, e, f, h and i) conditions and different nitrogen sources. Values represent the mean ± standard deviation (SD). An asterisk (*) indicates significant difference by t test (P ≤ 0.05, n = 4) between control and flooding or recovery plants for each
Oxidative stress in roots and leaves of plants under root flooding conditions
In leaves, O2·− generation rate increased in N2-fixing plants upon 48 h of flooding and in N2 + NO3− plants with 24 h of recovery. The decrease in O2·− was showed only by NO3− plants with 72 h of recovery in comparison to control plants (Fig. 5a). In roots, the generation rate of O2·− increased in N2-fixing plants upon 48 h of flooding and in N2 + NO3− with the return to normoxic conditions (24 and 72 h of recovery; Fig. 5b). The content of H2O2 increased upon 24 of recovery in leaves (Fig. 5c) and 72 h of recovery in roots (Fig. 5d). On the other hand, increases in ROS did not cause damages (lipid peroxidation) in leaves (Fig. 5e) and roots (Fig. 5f) in none of the treated plants.

source of nitrogen. NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants. a–b Superoxide content. c–d Hydrogen peroxide content. e–f Lipid peroxidation
Reactive oxygen species content (a–d) and lipid peroxidation (e and f) in leaves and roots of common bean plants subjected to flooding and recovery and different sources of nitrogen. Values represent log10 = (flood/control) or log10 = (recovery/control). An asterisk (*) indicates significant difference by t test (P ≤ 0.05, n = 4) between control and flooding or recovery plants for each
Antioxidative and glycolate oxidase enzyme activity under root flooding conditions
Glycolate oxidase (GO) activity decreased in leaves of N2-fixing plants upon 48 h of flooding and 24 h of recovery, while increased in NO3− and N2 + NO3− upon flooding and 24 h of recovery, respectively (Fig. 6a). Regarding the antioxidative enzyme system in leaves, N2-fixing plants decreased SOD and CAT and increased both APX and GPOD. In N2 + NO3− plants, there was a decrease in APX and GPOD upon 48 h of flooding. In NO3− plants increased CAT, APX, and GPOD upon flooding. GPOD decreased in all plants with 24 h of recovery (Fig. 6b). On the other hand, roots seem to be less efficient in inducing antioxidative system in relation to leaves, upon root flooding. All groups of common bean plants reduced the activity of SOD, CAT, APX, and GPOD upon flooding and recovery conditions (Fig. 6c).

source of nitrogen. NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants. Glycolate oxidase (GO), superoxide dismutase (SOD); catalase (CAT); ascorbate peroxidase (APX); guaiacol peroxidase (GPOD)
Heatmap of enzymatic activity in leaves (a and b) and roots (c) of common bean plants subjected to flooding and recovery and different sources of nitrogen. Variations of blue and red colors plotted as log10 = (flooding/control) or log10 = (recovery/control), indicate an increase and decrease, respectively, in the enzyme activity in plants under flooding or recovery in relation to the control. An asterisk (*) indicates significant difference by t test (P ≤ 0.05, n = 4) between control and flooding or recovery plants for each
Fermentative enzymes in roots upon flooding conditions
The activity of ADH increased substantially upon 48 h of flooding and did not decrease with 24 and 72 h of recovery in all treatments in comparison to control. In addition, the increase in N2-fixing plants was about twofold higher than plants which were supplied with nitrate (N2 + NO3− and NO3−) as shown in Fig. 7a–c. Similarly, LDH increased significantly in comparison to control in plants. The main increase was evidenced in N2-fixing plants, showing that nitrate reduced the effects of fermentation upon flooding and recovery. The activity of LDH, on the other hand, did not increase when nitrate-supplied plants (NO3−) were subjected to flooding conditions neither when normoxia was re-established (Fig. 7d–f).

source of nitrogen. Means followed by the same letters do not differ by Tukey’s test (P ≤ 0.05) among treatments (lowercase letter for control groups and capital letter for treated one). NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants. (n = 4). a–c Alcohol dehydrogenase (ADH). d–f Lactate dehydrogenase (LDH). (-) activity not detected
Fermentative enzyme activity in roots of common bean plants subjected to flooding and recovery and different sources of nitrogen. An asterisk (*) indicates significant difference by t test (P ≤ 0.05, n = 4) between control and flooding or recovery plants for each
The in vivo NR activity in plants of common bean
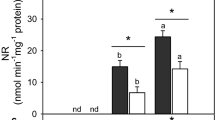
The NR enzyme activity in leaves increased significantly in nitrate-supplied plants (NO3−) and N2-fixing plants upon 48 h of flooding in comparison to control (Fig. 8a). On the other hand, no differences were observed in all treatments upon 24 h of recovery (Fig. 8b). In addition, the activity of NR decreased in N2-fixing and N2 + NO3− plants in comparison to control with 72 h of recovery (Fig. 8c). Furthermore, NR was higher in leaves of plants supplied with nitrate compared to N2-fixing plants upon 48 h of flooding and 24 h of recovery (Fig. 8a, b). In roots, an increase in NR activity was about 30- and 5-fold higher in plants supplied with nitrate (NO3− and N2 + NO3−, respectively) upon 48 h of flooding in comparison to control and to N2-fixing plants, remaining higher after 24 and 72 h of recovery. On the other hand, N2-fixing plants decreased NR activity upon 48 h of flooding and increased only with 72 h of recovery in comparison to control plants (Fig. 8d–f).

source of nitrogen. Means followed by the same letters do not differ by Tukey’s test (P ≤ 0.05) among treatments (lowercase letter for control groups and capital letter for treated one). NO3− = nitrate-supplied plants; N2 + NO3− = N2-fixing + pre-hypoxic nitrate-treated plants; N2 = nitrogen-fixing plants
Nitrate reductase (NR) activity in leaves (a–c) and roots (d–f) of common bean plants subjected to flooding and recovery and different sources of nitrogen. An asterisk (*) indicates significant difference by t test (P ≤ 0.05, n = 4) between control and flooding or recovery plants for each
Discussion
Several studies reported that exogenous supply of NO3−, but not NH4+ (including N2-fixing plants), exerts a positive effect on growth parameters, carbon and nitrogen metabolism in root cells (Thomas and Sodek 2005; Horchani et al., 2010; Oliveira et al. 2013a, b, c; Lanza et al. 2014; Wany et al. 2019). Here, we showed the involvement of nitrate in flooded roots of common bean plants and the possible extent effects on the shoot to maintain photosynthetic process by keeping the dynamic dissipation of the photosynthetic energy. Nitrate-supplied common bean (NO3−) and N2-fixing plants with pre-hypoxic supplementation of nitrate (N2 + NO3−) showed great maintenance of CO2 assimilation, gs and E which was not shown by N2-fixing plants (nitrate-free plants). In addition, no changes in the dynamic dissipation of photosynthetic energy were evidenced in nitrate-supplied plants upon flooding conditions. The nitrate-mediated maintenance of photosynthetic process may be related to increases in NR activity in roots of plants, which was reported to be associated with NO-cycle operating in the NAD+ recycling (van Dongen and Licausi 2015). Thus, NO-cycle avoids substantial increases in the activity of fermentative enzymes (ADH and LDH), and, consequently, reduces the deleterious effects of the main end-products of fermentation (Limami et al. 2014; Wany et al. 2019).
Oxygen deprivation increases glycolytic reactions (Pasteur effect) to keep low ATP production upon impairment of oxidative phosphorylation (Summers et al. 2000; Magneschi and Perata 2009; van Dongen and Licausi 2015; Pucciariello and Perata 2017). To keep glycolysis running, ADH and LDH were activated in N2-fixing plants to maintain cytosolic NAD+ recycling from NADH (Licausi 2011). However, the accumulation of end-products of fermentation (mainly lactic acid) (Zabalza et al. 2009), lead to cytosol acidification (Aroca et al. 2012), and aquaporin inhibition (Tournaire-Roux et al. 2003; Aroca et al. 2012). Consequently, water and nutrient uptake and transport from root to shoot may be limited, causing reductions in the photosynthetic rates of several species (Tournaire-Roux et al. 2003; Aroca et al. 2012; Herrera 2013; Bansal and Srivastava 2015; Posso et al. 2018). These effects may occur in N2-fixing plants, as evidenced by a reduction in E, gs and net assimilation rate under flooding. On the other hand, nitrate-supplied plants did not show any decrease in gas exchange upon root flooding.
In roots, NR has been reported to participate in a reaction known as NO cycle which converts NO3− to NO2− and the last one to NO (van Dongen and Licausi 2015), to regenerate NAD(P)+ and consume protons (Oliveira et al. 2013b; Limami et al. 2014; van Dongen and Licausi 2015; Wany et al. 2019). The increase in NR activity in nitrate-supplied plants competes for substrate (NADH) and reduces fermentative enzymes (ADH and LDH) (Zabalza et al. 2009; Pucciariello and Perata 2017). NO2− supplied to maize root segments was reported to decrease the cytoplasmic acidosis induced by hypoxia (Libourel et al. 2006).
The maintenance of cell homeostasis under flooding would help the root cells to uptake and translocate water and nutrients to the aerial part of the plants to maintain gas exchange, in NO3− and N2 + NO3− but not in N2-fixing plants. Lanza et al. (2014) have reported that NO3−-uptake by roots and its transport to the shoot occurs even under hypoxic conditions (Udvardi and Poole 2013), justifying the great increase in NR activity in leaf and root even under flooding conditions in nitrate-supplied plants. In addition, the increase in NR activity in leaves of N2-fixing plants under root flooding conditions is justified due to the presence of nitrate in the tissues, as a result of plant cell metabolism (Lanza et al. 2014).
The maintenance of CO2 assimilation by nitrate-supplied plants are supported by the maintenance of the dynamic energy dissipation of the photosynthetic energy through the evaluation of the Chl a fluorescence, which is not observed in N2-fixing plants. N2-fixing plants showed increased accumulation of QA− (OJ-phase), high energy dissipation as heat (JI-phase), and high fluorescence emission (Wt) upon flooding, which is kept even upon 7 days after re-oxygenation. The increase in energy dissipation (non-photochemical quenching) is related to a block in ETC that impairs the electron flow further than QA− (Strasser et al. 2004; Yusuf et al. 2010), Although flooding increased fluorescence emission in N2 + NO3− plants, the dynamic of energy dissipation was re-established upon recovery of plants.
Negative effects of flooding were also observed upon recovery of N2-fixing plants with an increase in specific energy fluxes per RC and decreases in quantum yields and efficiencies. These plants showed a reduction in quantum yield for electron transport (φEo), quantum yield for reduction of end electron acceptors at the PSI acceptor side (φRo), efficiency/probability for electron transport, i.e., efficiency/probability that an electron moves further than QA− (ψEo), as a result of the blockage in the ETC. On the other hand, an impairment in electron transport at the QA− site increases the efficiency/probability of the electron flow further than QA− reaches the PSI acceptor side though it did not increase RE0/RC (reduce end electron acceptors at the PSI acceptor side per RC), which might lead to a lower NADPH and ATP production (Strasser and Strasser 1995; Strasser et al. 2004; Yusuf et al. 2010).
The maintenance of PIABS and PItotal in NO3− and N2 + NO3− plants upon recovery correlated to the reaction center functionality, efficiency in energy absorption and electron transport between the photosystems, which demonstrate the great functionality of the ETC in comparison to N2-fixing plants. On the other hand, Tsmilli-Michael and Strasser (2008) reported that an increase in the sequence of events from exciton trapping by PSII up to plastoquinone (PQ) reduction (OI-phase; WOI from 0 to 1) and a decline in the reduction of the end electron acceptors on the PSI acceptor side (IP-phases; WOI ≥ 1) support the evidence of a low NADPH and ATP production that leads to a decrease in CO2 assimilation in N2-fixing plants. In addition, plants showed lower energy connectivity of PSII (indicated by the presence of the positive L-band), upon flooding. Contrastingly, N2 + NO3− plants re-established efficiently the connectivity of PSII upon recovery, while flooding did not affect system stability and energy utilization of NO3− plants, the efficient energy transfer between PSII units, due to the absorption (Tsimilli-Michael and Strasser 2008), and decrease in energy dissipation (non-photochemical quenching) as heat and fluorescence (Stirbet and Govindjee 2011; Santos Junior et al. 2015).
Moreover, the appearance of a positive K-band in N2-fixing plants demonstrates an inactivation of the OEC and an increase in PSII antenna size (Yan et al. 2013), which causes an imbalance in the energy flux in PSII and increases the probability of photooxidative damage (Foyer et al. 2017), supported by an increase in ABS/RC. The maintenance of photosynthetic energy flow may maintain the redox state for CO2 assimilation by Rubisco (Ahmed et al. 2002; Bansal and Srivastava 2015) in nitrate-supplied plants. In addition, CO2 uptake is kept by the maintenance of gs and E, possible through water absorption by roots (Bansal and Srivastava 2015) and its transport through xylem sap to the shoot (Aroca et al. 2012; Herrera 2013). Alternatively, the reducing power and adenylate energy charge can be efficiently dissipated via photorespiration (increase in GO activity), in NO3− plants, while it does not operate in N2-fixing plants as evidenced by a decrease in GO activity. It would avoid ROS production by alleviating ETC from an over-reduction (Bräutigam and Gowik 2016).
The increase in TR0/RC and the presence of positive L- and K-band demonstrate an impairment in ETC in N2-fixing plants that may contribute to electron leakage and ROS production, evidenced by higher O2·− generation rate in these plants. The higher O2·− generation rate was a result of an inefficient activation of SOD, which is considered the first defence line in scavenging the O2·− (Blokhina and Fagerstedt 2010a, b; Mitler 2017). Moreover, the increase in APX activity, an enzyme that scavenges H2O2, may maintain its production low in the cells. An efficient antioxidative enzyme system induction avoids triggering oxidative damage to lipid membrane and lipid peroxidation, protein, DNA and RNA degradation and enzyme inactivation (Gill and Tuteja 2010). The higher increase in APX activity in leaves of N2-fixing plants, but not CAT, to scavenge H2O2 might be to help the dissipation of reducing power, a co-factor of APX in the ascorbate–glutathione cycle (Blokhina and Fagerstedt 2010b, a; Mitler 2017). On the other hand, nitrate-supplied plants under flooding did not increase ROS production and lipid peroxidation in comparison to its respective control. In addition, NO3− plants efficiently increased the induction of the enzymes CAT, APX, and GPOD to counteract with possible effects of ROS production in leaves.
In roots, the activity of mitochondrial ETC became limited upon flooding due to a reduction in electron transport through the complexes which declines electron leakage and, consequently ROS production (Azevedo Neto et al. 2006; Bai et al. 2014). In leaves, however, ETC is driven by light absorption. Besides that, nitrate also plays an important role in another pathway after its reduction to nitrite by NR itself. Nitrite is converted to NO via the complex IV (cytochrome c oxidase; COX), allowing proton translocation (Gupta et al. 2005, 2011; Gupta and Igamberdiev 2011; Oliveira et al. 2013b). NO is then oxidized to nitrate again by nsHb (Igamberdiev and Hill 2004), which would help the maintenance of mitochondrial ETC under flooding conditions (Wany et al. 2019), and low ATP production under hypoxia (Horchani et al. 2010). This may explain the increase of O2·− generation rate in roots of N2-fixing plants upon flooding.
Upon flooding conditions, NO is produced from nitrite reduction only in root cells (Gupta et al. 2005). Although nitrate is transported in low concentrations to the shoot through xylem sap from roots (Oliveira et al. 2013a; Lanza et al. 2014), even with high NR activity in leaves, the lacking ability of leaf mitochondria and NR enzyme in the cytosol to produce NO may somehow be limited due to photosynthesis and the aerobic conditions of the leaves (Gupta et al. 2005), which induces differential response between roots and leaves, regarding ROS production and antioxidative enzyme induction. The beneficial effects of the NO-cycle (cytosol and mitochondria) of roots may lead to the maintenance of ROS production upon flooding, which is not observed in N2-fixing plants, though further investigations are needed. The low production of ROS in roots also leads to a low induction of antioxidative enzyme system, which is not observed in leaves.
Conclusion
The involvement of nitrate in common bean plants under flooding conditions induces significant increases in NR activity in roots, which may play a role in glycolysis regulation to alleviate the toxic effects of end-products through the decrease of fermentative enzyme activity. The release of possible deleterious root cell effects may help plants to keep the E and gs to maintain photosynthetic process operating with no changes in the dynamic energy dissipation in leaves of common bean plants, mediated by nitrate in roots under flooding conditions.
Author contribution statement
D.A.P., J.B. and M.A.B.: conception and design of the experiment. D.A.P., J.B., and G.N.R.: data acquisition. D.A.P., J.B., G.N.R., L.A., and M.A.B.: data analysis. All author contributed to drafting and revising the manuscript.
References
Ahmed S, Nawata E, Sakuratani T (2002) Effects of waterlogging at vegetative and reproductive growth stages on photosynthesis, leaf water potential and yield in mungbean. Plant Prod Sci 5:117–123. https://doi.org/10.1626/pps.5.117
Amarante L, Lima JD, Sodek L (2006) Growth and stress conditions cause similar changes in xylem amino acids for different legume species. Environ Exp Bot 58:123–129
Armstrong W, StrangeE ME, Cringles S, Beckett PM (1994) Microelectrode and modeling study of oxygen distribution in roots. Ann Bot 74:287–299. https://doi.org/10.1006/anbo.1994.1120
Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63:43–57. https://doi.org/10.1093/jxb/err2
Azevedo Neto AD, Prisco JT, Eneas Filho J, De Abreu CEB, Gomes Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94. https://doi.org/10.1016/j.envexpbot.2005.01.008
Bai YR, Yang P, Su YY, He ZL, Ti XN (2014) Effect of exogenous methanol on glycolate oxidase and photorespiratory intermediates in cotton. J Exp Bot 65:5331–5338. https://doi.org/10.1093/jxb/eru294
Bailey-Serres J, Fukao T, Gibbs DJ, Holsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17:129–138. https://doi.org/10.1016/j.tplants.2011.12.004
Bansal R, Srivastava JP (2015) Effect of waterlogging on photosynthetic and biochemical parameters in pigeonpea. Russ J Plant Physiol 62:322–327. https://doi.org/10.1134/S1021443715030036
Blokhina O, Fagerstedt KV (2010a) Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol Biochem 48:359–373. https://doi.org/10.1016/j.plaphy.2010.01.007
Blokhina O, Fagerstedt KV (2010b) Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems. Physiol Plant 138:447–462. https://doi.org/10.1111/j.1399-3054.2009.01340.x
Borella J, Oliveira HC, de Oliveira DDSC, Braga EJB, Oliveira ACB, Sodek L, do Amarante L (2017) Hypoxia-driven changes in glycolytic and tricarboxylic acid cycle metabolites of two nodulated soybean genotypes. Environ Exp Bot 133:118–127. https://doi.org/10.1016/j.envexpbot.2016.10.007
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bräutigam A, Gowik U (2016) Photorespiration connects C3 and C4 photosynthesis. J Exp Bot 67:2953–2962. https://doi.org/10.1093/jxb/erw056
Cakmak I, Horst WJ (1991) Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468. https://doi.org/10.1111/j.1399-3054.1991.tb00121.x
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. https://doi.org/10.1016/0003-2697(76)90488-7
Foyer CH, Ruban AV, Noctor G (2017) Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem J 474:877–883. https://doi.org/10.1042/BCJ20160814
Garcia N, da Silva CJ, Cocco KLT, Pomagualli D, Oliveira FK, Silva JVL, Oliveira ACB, Amarante L (2020) Waterlogging tolerance of five soybean genotypes through different physiological and biochemical mechanisms. Environ Exp Bot 172:1–8. https://doi.org/10.1016/j.envexpbot.2020.103975
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gupta KJ, Igamberdiev AU (2011) The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 11:537–543. https://doi.org/10.1016/j.mito.2011.03.005
Gupta KJ, Igamberdiev AU, Manjunatha G, Segu S, Moran JF, Neelawarne B, Bauwe H, Kaiser WM (2011) The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci 181:520–526. https://doi.org/10.1016/j.plantsci.2011.03.018
Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56:2601–2609. https://doi.org/10.1093/jxb/eri252
Hageman RH, Reed AJ (1980) Nitrate reductase from higher plants. Methods Enzymol 69:270–280. https://doi.org/10.1016/S0076-6879(80)69026-0
Hanawa H, Ishizaki K, Nohira K, Takagi D, Shimakawa G, Takehiro S, Shaku K, Makino A, Miyake C (2017) Land plants drive photorespiration as higher electron-sink: comparative study of post-illumination transient O2-uptake rates from liverworts to angiosperms through ferns and gymnosperms. Physiol Plant 161:138–149. https://doi.org/10.1111/ppl.12580
Hanson AD, Jacobsen JV (1984) Control of Lactate dehydrogenase, lactate glycolysis, and α-amylase by O2 deficit in barley aleurone layers. Plant Physiol 74:566–572
Hanson A, Jacobsen J, Zwar J (1984) Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Pl Physiol 75(3):573–581
Herrera A (2013) Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front Plant Sci 4:1–12. https://doi.org/10.3389/fpls.2013.00106
Hoagland DR, Arnon DI (1938) The water culture method of growing plants without soil. Cal Agri Exp Sta 347:1–39
Horchani F, Aschi-Smiti S, Brouquisse R (2010) Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) to prolonged root hypoxia. Acta Physiol Plant 32:1113–1123. https://doi.org/10.1007/s11738-010-0503-0
Ivanov BN, Borisova-Mubarakhina MM, Kozuleva MA (2018) Formation mechanisms of superoxide radical and hydrogen peroxide in chloroplasts, and factors determining the signalling by hydrogen peroxide. Funct Plant Biol 45:102–110. https://doi.org/10.1071/FP16322
Jackson MB, Colmer TD (2005) Response and adaptation by plants toflooding stress. Ann Bot 96:501–505. https://doi.org/10.1093/aob/mci205
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279. https://doi.org/10.1016/S0006-291X(71)80010-4
Justino GC, Sodek L (2013) Recovery of nitrogen fixation after short-term flooding of the nodulated root system of soybean. J Plant Physiol 170:235–241. https://doi.org/10.1016/j.jplph.2012.10.006
Lanza LNM, Lanza DCF, Sodek L (2014) Utilization of 15NO3—by nodulated soybean plants under conditions of root hypoxia. Physiol Mol Biol Plant 20:287–293. https://doi.org/10.1007/s12298-014-0241-7
Li C, Bai T, Ma F, Han M (2010) Hypoxia tolerance and adaptation of anaerobic respiration to hypoxia stress in two Malus species. Sci Hort 124:274–279. https://doi.org/10.1016/j.scienta.2009.12.029
Libourel IGL, van Bodegom PM, Fricker MD, Ratcliffe RD (2006) Nitrite reduces cytoplasmic acidosis under anoxia. Plant Physiol 142:1710–1717. https://doi.org/10.1104/pp.106.088898
Licausi F (2011) Regulation of the molecular response to oxygen limitations in plants. New Phytol 190:550–555. https://doi.org/10.1111/j.1469-8137.2010.03562.x
Limami A, Diab H, Lothier J (2014) Nitrogen metabolism in plants under low oxygen stress. Planta 239:531–541. https://doi.org/10.1007/s00425-013-2015-9
Loureiro MF, James EK, Franco AA (1998) Nitrogen fixation by legumes in flooded regions. In: Franco AC, Scarano FR (eds) Ecophysiological strategies of xerophytic and amphibious plants in the neotropics. Series Oecologia Brasiliensis, Brazil, pp 195–233
Magneschi L, Perata P (2009) Rice germination and seedlings growth in the absence of oxygen. Ann Bot 103:181–196. https://doi.org/10.1093/aob/mcn121
Mitler R (2017) ROS are good. Trend. Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–17. https://doi.org/10.1042/BJ20081386
Oliveira HC, Freschi L, Sodek L (2013a) Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiol Biochem 66:141–149. https://doi.org/10.1016/j.plaphy.2013.02.015
Oliveira HC, Salgado I, Sodek L (2013b) Involvement of nitrite in the nitrate-mediated modulation of fermentative metabolism and nitric oxide production of soybean roots during hypoxia. Planta 237:255–264. https://doi.org/10.1007/s00425-012-1773-0
Oliveira HC, Salgado I, Sodek L (2013c) Nitrite decreases ethanol production by intact soybean roots submitted to oxygen deficiency. Plant Sig Beh 8(4):e23578. https://doi.org/10.4161/psb.23578
Osorno J, Endres G, Ashley R, Kandel H, Berglund D (2014) Dry bean production guide. NDSU Agriculture, Fargo. 17p. http://www.ag.ndsu.edu/pubs/plantsci/rowcrops/a1133-1.htm. https://library.ndsu.edu/ir/bitstream/handle/10365/9510/a1133_1997.pdf?sequence=1&isAllowed=y. https://www.ag.ndsu.edu/publications/crops/dry-bean-production-guide
Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA (2010) Distinct signalling pathways and transcriptome response signatures differentiate ammonium-and nitrate-supplied plants. Plant Cell Environ 33:1486–1501. https://doi.org/10.1111/j.1365-3040.2010.02158.x
Posso DA, Borella J, Reissig GN, Bacarin MA (2018) Root flooding-induced changes in the dynamic dissipation of the photosynthetic energy of common bean plants. Acta Physiol Plant 40:212. https://doi.org/10.1007/s11738-018-2790-9
Pucciariello C, Perata P (2017) New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ 40:473–482. https://doi.org/10.1111/pce.12715
Sánchez C, Tortosa G, Granados A, Delgado A, Bedmar EJ, Delgado MJ (2011) Involvement of Bradyrhizobium japonicum denitrification in symbiotic nitrogen fixation by soybean plants subjected to flooding. Soil Biol Biochem 43:212–217. https://doi.org/10.1016/j.soilbio.2010.09.020
Santos UM Jr, Gonçalves JFC, Strasser RJ, Fearnside PM (2015) Flooding of tropical forests in central Amazonia: what do the effects on the photosynthetic apparatus of trees tell us about species suitability for reforestation in extreme environments created by hydroelectric dams? Acta Physiol Plant 37:1–17. https://doi.org/10.1007/s11738-015-1915-7
StirbetGovindjee A (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications. J Photochem Photobiol B: Biol 104:236–257. https://doi.org/10.1016/j.jphotobiol.2010.12.010
Stoimenova M, Libourel IGL, Ratcliffe RG, Kaiser WM (2003) The role of nitrate reduction in the anoxic metabolism of roots I. Characterization of root morphology and normoxic metabolism of wild type tobacco and a transformant lacking root nitrate reductase. Plant Soil 253:155–167. https://doi.org/10.1023/A:1024563507275
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P (ed) Photosynthesis: from light to biosphere. Montpellier, France, pp 977–980
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence a signature of photosynthesis. Springer, Dordrecht, pp 321–362
Summers J, Ratcliffe R, Jackson M (2000) Anoxia tolerance in the aquatic monocot Potamogeton pectinatus: Absence of oxygen stimulates elongation in association with an unusually large Pasteur effect. J Exp Bot 51(349):1413–1422
Thomas AL, Sodek L (2005) Development of nodulated soybean plant after flooding of the root system with different sources of nitrogen. Braz J Plant Physiol 17:291–297. https://doi.org/10.1590/S1677-04202005000300003
Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425:393–397. https://doi.org/10.1038/nature01853
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of plants vitality: applications in detecting and evaluating the impact of Mycorrhization on host plants. In: Varma A (ed) Mycorrhiza, 3rd edn. Springer, Uttar Pradesh, pp 679–703
Udvardi M, Poole PS (2013) Transport and metabolism in legume-rhizobia symbioses. Ann Rev Plant Biol 64:781–805. https://doi.org/10.1146/annurev-arplant-050312-120235
United States Department of Agriculture (USDA) (2015) Hearing: agriculture’s role in combatting global hunger. Government Publishing Office, Washington, U.S, p 126
van Dongen JT, Licausi F (2015) Oxygen sensing and signalling. Ann Rev Plant Biol 66:345–367. https://doi.org/10.1146/annurev-arplant-043014-114813
Velikova V, Yordanov I, Eedreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387. https://doi.org/10.1007/BF00384257
Wany A, Gupta AK, Kumari A, Mishra S, Singh N, Pandey S, Vanvari R, Igamberdiev AU, Fernie AF, Gupta KG (2019) Nitrate nutrition influences multiple factors in order to increase energy efficiency under hypoxia in Arabidopsis. Ann Bot 123:691–705. https://doi.org/10.1093/aob/mcy202
Yan K, Chen P, Shao H, Shao C, Zhao S, Brestic M (2013) Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE. https://doi.org/10.1371/journal.pone.0062100
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee SNB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Bioch Biophys Acta (BBA)-Bioenerg 1797:1428–1438. https://doi.org/10.1016/j.bbabio.2010.02.002
Zabalza A, van Dongen JT, Froehlich A, Oliver SN, Faix B, Gupta KJ, Schmälzlin E, Igal M, Orcaray L, Royuela M, Geigenberger P (2009) Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol 149:1087–1098. https://doi.org/10.1104/pp.108.129288
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES)-Finance Code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We are grateful to Embrapa-Terras Baixas and Stoller® for kindly providing common bean seeds and Rhizobium tropici strain, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Esposito.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Posso, D.A., Borella, J., Reissig, G.N. et al. Nitrate-mediated maintenance of photosynthetic process by modulating hypoxic metabolism of common bean plants. Acta Physiol Plant 42, 117 (2020). https://doi.org/10.1007/s11738-020-03107-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03107-y