Abstract
p16 is generally considered to be a surrogate maker of human papillomavirus (HPV) infection and also a predictive marker of favorable clinical outcome of patients with squamous cell carcinoma of the oropharynx. p16 overexpression is also known to be induced by deregulation of RB1 in neuroendocrine carcinomas. In highly malignant esophageal neoplasms, however, the status of p16 has remained largely unknown. We immunolocalized p16 and Rb1 in 82 surgically resected esophageal high-grade squamous cell carcinomas (46 poorly differentiated and 36 basaloid squamous cell carcinomas) and 15 esophageal small-cell carcinomas in order to clarify the clinical and biological significance of p16. p16 immunoreactivity was detected in 7/82 (9%) high-grade squamous cell carcinomas and 15 (100%) small-cell carcinomas. p16 immunoreactivity was significantly associated with Rb1 protein loss in both groups (P < 0.001). HPV was detected in none of the p16-positive cases examined. Clinical outcome of the p16-positive high-grade squamous cell carcinomas was not different from that of the p16-negative counterparts (P = 0.687) but significantly better than those with the small-cell carcinomas (P = 0.023). p16 was therefore considered to be induced through an inactivation of the RB1 signaling pathway and not through HPV infection in highly malignant esophageal neoplasms. Nevertheless, patients’ clinical outcome of these neoplasms significantly differs; therefore, small-cell carcinomas have to be carefully differentiated from other neoplasms. In addition, p16 overexpression is not predictive of favorable clinical outcome in high-grade squamous cell carcinomas of the esophagus.
Similar content being viewed by others
Introduction
Approximately 85% of esophageal neoplasms are squamous cell carcinomas in East Asian countries [1, 2]. Among these patients, histological differentiation and types are associated with clinical outcome of the patients; in particular, poorly differentiated or basaloid squamous cell carcinomas of the esophagus have significantly worse prognosis than well- to moderately differentiated ones [3,4,5]. In addition, esophageal small-cell carcinomas, which account for 0.05–3.1% of all esophageal malignancies [1, 6, 7], are known to be highly aggressive [3, 6, 8].
p16 is a protein product of tumor suppressor gene CDKN2A and a member of the INK4 family of cyclin-dependent kinase inhibitors. p16 binds to and inactivates cyclin-dependent kinases 4/6, resulting in the suppression of the phosphorylation of retinoblastoma 1 (Rb1) protein (a tumor suppressor protein) and cell cycle progression [9, 10]. In general, CDKN2A is frequently inactivated in human malignant tumors by its gene aberrations, such as deletions or mutations [9, 10], but diffuse p16 expression was also reported in various human malignancies, e.g., high-grade breast and lung cancers and/or undifferentiated pleomorphic sarcoma without significant association with its gene alteration status [9, 11]. In these neoplasms, p16 was reported to be induced by deregulation of RB1 as positive feedback [9, 12]. The p16 overexpression via RB1 alteration was on the one hand demonstrated in highly aggressive tumors, such as small-cell carcinomas of the lung and esophagus [12,13,14]. In particular, p16 overexpression and loss of Rb1 protein were constantly detected in esophageal small-cell carcinomas [12].
On the other hand, p16 protein overexpression is also considered a surrogate marker of oncogenic human papillomavirus (HPV) infection. HPV infection plays a pivotal role in carcinogenesis of oropharyngeal, uterine cervical, and perianal cancers [9, 15, 16]. Following the entry of virion via endocytosis, the virus establishes a persistent infection as a viral episome or integrates into the host genome [17]. HPV E6 and E7 oncoproteins are expressed from both forms of the viral DNA, which could inactivate tumor suppressors p53 and Rb1 proteins and lead to cell cycle progression and subsequently to development of cancer [17, 18]. Consequently, an inactivation of Rb1 protein results in the overexpression of p16 [9, 12]. HPV-associated oropharyngeal squamous cell carcinomas were pathologically and clinically different from the non-HPV-associated counterparts [19]. These tumors had a lower metastatic rate and TNM stages than HPV-negative squamous cell carcinomas [19], resulting in better clinical outcome and response to chemotherapy, radiotherapy, or chemoradiotherapy than those with HPV-negative squamous cell carcinomas [20,21,22,23]. Therefore, an evaluation of p16 status was proposed essential in the diagnosis of oropharyngeal malignancy [24]. At this juncture, p16-positive oropharyngeal squamous cell carcinomas are separately staged from those without p16 expression [24]. In the esophagus, HPV-positive squamous cell carcinomas were also reported, but its reported incidence enormously varied: 0–63% in Japan, 1–77% in China, 8–74% in India, 0–41% in Europe, and 0–5% in the USA [25,26,27,28]. In addition, the patients with p16-/HPV-positive squamous cell carcinoma of the esophagus were reported to be associated with better clinical outcome [28, 29] and respond better to chemoradiotherapy than the p16-/HPV-negative patients [30]. However, the status of p16 and its correlation with HPV infection in highly malignant neoplasms of the esophagus have remained largely unknown.
In our present study, we aimed to clarify (i) the status of p16 and its relation with HPV infection, (ii) the clinicopathological characteristics of p16-positive tumors in comparison with p16-negative tumors, and (iii) the clinical outcome of the corresponding patients in highly malignant neoplasms of the esophagus including poorly differentiated and basaloid squamous cell carcinomas, as well as esophageal small-cell carcinomas.
Materials and methods
Tumor specimens
We studied 97 esophageal carcinoma cases: 15 small-cell carcinomas and 82 high-grade squamous cell carcinomas. The high-grade squamous cell carcinomas, termed based on their aggressive clinical nature, consisted of 46 poorly differentiated and 36 basaloid squamous cell carcinomas [3, 5]. All the specimens were surgically resected between 1988 and 2017. The specimens of esophageal small-cell carcinoma were retrieved from six Japanese institutions listed in a previous study [6], and high-grade squamous cell carcinomas from a single institution (Tohoku University Hospital, Sendai, Japan). The clinical data of the patients were reported in the previous studies to compare the outcomes of small-cell carcinoma and basaloid squamous cell carcinoma patients, as well as SOX2 and Rb1 expression in small-cell carcinomas [3, 6]. All the specimens were independently reviewed by three pathologists (AK, FF, and HS). The histological diagnosis of small-cell carcinoma was made based on the histomorphology and presence of immunoreaction of at least one of synaptophysin and chromogranin A [6, 31]. Pathological diagnosis of high-grade squamous cell carcinoma was made based on histomorphology and presence of positive staining for p40 by immunohistochemistry as previously described [3, 6, 31]. Tumors with the following conditions were excluded: (i) located at the esophagogastric junction or concomitant Barrett’s esophagus and (ii) eradication of more than two thirds of the tumor with preoperative therapy [6].
Clinical information of all the patients examined was carefully reviewed. TNM staging was determined according to the eighth edition of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system for esophageal carcinoma [24]. Disease-specific survival was defined as the time from initial pathological diagnosis to disease-related death or last observation. The study protocol was approved by the ethics committees of all participating institutions.
Immunohistochemical analysis
Specimen preparation and immunohistochemical stainings of synaptophysin, chromogranin A, p40, and Rb1 were performed as previously described [6]. Anti-p16 monoclonal antibody (clone G175-1239; dilution 1:100, pH 6.0 buffer) was commercially obtained from BD Pharmingen, NJ, USA. The above-mentioned immunohistochemistry was performed in all cases included in this study. Labeling index against the nuclear antigens p40, p16, and Rb1 was all quantified using the HistoQuest semi-automated image analysis software (TissueGnostics, Tarzana, Los Angeles, USA) in an area of 0.04 mm2, and the percentage of immunopositive nuclei in total tumor cells was subsequently obtained [6, 32]. In evaluating synaptophysin and chromogranin A immunoreactivity, a well-established immunoreactivity scoring system ranging 0–12 was employed. The score was calculated by multiplying the positivity (0, 0%; 1, < 10%; 2, 10–50%; 3, 51–80%; or 4, > 80% of tumor cells positively stained) and the staining intensity (0, none; 1, mild; 2, moderate; or 3, strong staining), and positive immunoreactivity was defined as a final score of ≥ 1 and negative as a final score of 0, as previously described [6, 33]. Positive immunoreactivity of p40 and p16 was defined as their labeling index of ≥ 1% and negative as index < 1% [6, 33]. Positive (normal) immunoreactivity of Rb1 was defined as its labeling index of ≥ 10% and negative (loss) as index < 10% [34]. Reactive stromal cells, lymphocytes, and/or non-neoplastic epithelial cells were used as positive internal controls for p16 and Rb1 (Supplementary Fig. 1). The evaluation methods are summarized in Supplementary Table 1.
DNA extraction from 10% formalin-fixed, paraffin-embedded blocks
In order to explore the status of HPV infection in p16-positive tumors, genomic DNA of both esophageal small-cell carcinoma and high-grade squamous cell carcinoma cases was selectively extracted from the tumorous area of unstained tissue sections (thickness, 10 μm × 5 sections) under the guidance of serial hematoxylin and eosin-stained tissue sections using a QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol [35]. The quality of the extracted DNA was confirmed by polymerase chain reaction (PCR) using a beta-globin (human) Primer Set (TaKaRa Bio., Shiga, Japan) according to the manufacturer’s instructions [36]. An amplified DNA sample via PCR was considered as an appropriate sample for the following PCR experiment.
Polymerase chain reaction for typing human papillomavirus
PCR was performed in samples using a Light Cycler equipment (Roche, Basel, Switzerland) and commercially available HPV typing set (TaKaRa Bio., Shiga, Japan) to identify HPV genotypes 6, 11, 16, 18, 31, 33, 35, 52, and 58 in genomic DNA with specifically designed primer sets and according to the manufacturer’s instructions [37]. Other rare genotypes of HPV were not detectable by this approach. The primer sequences used were as follows: HPV pU-31B forward for low-risk HPV genotypes 6 and 11, 5′-TGCTAATTCGGTGCTACCTG-3′; HPV pU-1M forward for high-risk HPV genotypes 16, 18, 31, 33, 35, 52, and 58, 5′-TGTCAAAAACCGTTGTGTCC-3′; and HPV pU-2R reverse, 5′-GAGCTGTCGCTTAATTGCTC-3′. This primer set included both malignant and benign control templates for verification of the PCR results using the primer pairs provided in the set. The PCR products were evaluated by 2.0% agarose gel electrophoresis stained with ethidium bromide in Tris–borate–ethylenediaminetetraacetic acid buffer.
Statistical analyses
JMP Pro version 13.0.0 software (SAS Institute, Inc., Cary, NC, USA) was used for all statistical analyses. Continuous data were analyzed using Student’s t test or the Mann–Whitney U test. Correlations between two variables were identified using Pearson’s chi-square test, Fisher’s exact test, or Mann–Whitney U test as appropriate. Disease-specific survival curves were constructed using the Kaplan–Meier method, and compared using the log-rank test. A P value of < 0.05 was considered statistically significant.
Results
Clinicopathological features
The clinicopathological characteristics of the 97 esophageal neoplasms examined in this study are summarized in Supplementary Table 2. There were no significant differences in age, gender, tumor location, and tumor size between small-cell and high-grade squamous cell carcinomas or between poorly differentiated and basaloid squamous cell carcinomas (data not shown). The TNM stage of small-cell carcinoma was higher than that of high-grade squamous cell carcinoma (P = 0.018, data not shown).
Immunohistochemical characteristics
The results of the immunohistochemistry analysis of esophageal small-cell and high-grade squamous cell carcinomas are summarized in Table 1. Synaptophysin immunoreactivity was detected in all esophageal small-cell carcinomas, whereas chromogranin A was expressed in 10 (67%) esophageal small-cell carcinomas. Expression of synaptophysin and chromogranin A was not detected in any of the high-grade squamous cell carcinoma specimens. p16 expression was detected in all the small-cell carcinomas (n = 15, 100%). The immunoreactivity was observed mostly in nuclei and cytoplasm of the tumor cells with strong intensity and consistent high labeling index (range, 63–93%; mean, 83%; Fig. 1). In the high-grade squamous cell carcinomas, p16 immunoreactivity was focally detected in nuclei and cytoplasm with strong intensity in 7/82 cases (9%) (Fig. 2). The mean labeling index of the cases with positive p16 immunoreaction (7 cases) was 33%, ranging from 7% to 80% (Fig. 3). The p16 labeling index was also significantly higher in the esophageal small-cell carcinomas than in the poorly differentiated squamous cell carcinomas, as well as the basaloid squamous cell carcinomas (P < 0.001 for both) (Table 1). No significant difference was detected in p16 status between the poorly differentiated squamous cell (mean index 2% in all cases, 4/46 positive) and the basaloid squamous cell carcinomas (mean index 3% in all cases, 3/36 positive). All esophageal small-cell carcinomas exhibited diffuse and uniformly strong immunopositivity of p16, whereas high-grade squamous cell carcinomas demonstrated a diffuse and strong pattern in one case (1%), a focal positivity in 5 cases (6%), and a single-cell positivity in one case (1%). Loss of Rb1 protein expression was detected in all the 15 small-cell carcinomas (Figs. 1 and 3) and 12/82 of the high-grade squamous cell carcinomas (15%) (Figs. 2 and 3). In the high-grade squamous cell carcinomas, the status of p16 immunoreactivity was significantly associated with female gender (P = 0.047) but not with age, location, size, TNM stage, or histological type (Supplementary Table 3). The labeling indexes of p16 and Rb1 were inversely correlated with each other (P < 0.001, Fig. 3). In order to test whether the used cut-off level of 1% for determination of p16 expression status is appropriate to correlate Rb1 loss, we also tested different values (from 5% to 35%) but they did not result in different statistical correlations. Among the p16-positive high-grade squamous cell carcinomas (n = 7), 4 with a relatively high labeling index of p16 (index, 80, 43, 41, and 38%) demonstrated no Rb1 protein expression (index, all 0%) and presented features related to aggressive biological behavior: tumor size ≥ 50 mm, lymph node involvement, and a high TNM stage (III/IV). In contrast, the other 3 with a low labeling index of p16 (index, 7, 11, and 14%) demonstrated indolent clinical characteristics: tumor size < 50 mm, no lymph node metastasis, and a low TNM stage (I/II). Two out of the 3 had normal Rb1 protein expression (index, 74 and 46%) (Fig. 3).
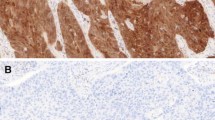
Histopathological and immunohistochemical features of esophageal small-cell carcinoma. The tumor with variably sized solid nests with ill border. Abundant intratumoral necrosis (yellow arrows, a). Tumor cells are round to oval in shape with hyperchromatic nuclei and scant cytoplasm (b). Diffuse nuclear and cytoplasmic p16 immunoreactivity (c) and loss of Rb1 protein in the carcinoma cells (d). No amplification of human papillomavirus DNA of a small-cell carcinoma tissue and a control tissue showing a positive band by polymerase chain reaction (e)
Histopathological and immunohistochemical features of poorly differentiated squamous cell carcinoma of the esophagus. Tumor cells of poorly differentiated squamous cell carcinoma comprised round cells without apparent keratinization (a and b). Immunoreactivity of p16 was expressed in 7 out of 82 cases (c), while loss of Rb1 protein was detected in 12 out of 82 cases of high-grade squamous cell carcinoma (d). No amplification of human papillomavirus DNA (e)
All the small-cell carcinomas (plotted in white circles) demonstrated high immunoreactivity of p16 and loss of Rb1 protein expression. In contrast, most of the poorly differentiated (black points) and basaloid (black triangles) squamous cell carcinomas showed no p16 overexpression and normal Rb1 expression. p16 expression and loss of Rb1 expression were observed in 4 and 3 cases of poorly differentiated and basaloid squamous cell carcinomas, respectively. The p16 expression was significantly associated with the loss of Rb1 protein (P < 0.001)
Marked and diffuse immunoreactivity of p40 was detected in all the high-grade squamous cell carcinomas (100%, 82/82 cases) with a mean index of 78%, whereas p40 was negative in 14 small-cell carcinomas. The other one small-cell carcinoma (7%, 1/15 case) presented weak p40 immunoreactivity.
We confirmed the accuracy of staining using controls’ tissues listed in Supplementary Table 1.
Human papillomavirus status in p16-expressing neoplasms
DNA were extracted from 3 of the small-cell carcinomas with the highest labeling index of p16 (93, 92, and 91%) and all the high-grade squamous cell carcinomas with positive p16 expression. The extracted DNA was amplified using beta-globin (human) Primer Set. DNA amplification was not successful in one of the high-grade squamous cell carcinomas probably due to insufficient purity of the extracted DNA. We conducted PCR using the amplified DNA for detection of HPV infection. In both malignant control (63 bp) and benign control (61 bp) templates, the DNA was amplified and PCR was performed successfully. No amplification of HPV DNA regardless of tissue subtypes was detected in esophageal small-cell and high-grade squamous cell carcinomas (Figs. 1e and 2e).
Clinical outcome
The 5-year disease-specific survival of the patients with esophageal small-cell carcinoma was 7% with a median survival time of 15 months. The 5-year disease-specific survival of the patients with high-grade squamous cell carcinoma of the esophagus was 57% (median survival time, 92 months). The survival rate of the esophageal small-cell carcinoma patients was significantly worse than that of the high-grade squamous cell carcinoma patients (P < 0.001, Fig. 4). Clinical outcome of the patients with basaloid squamous cell carcinoma (5-year disease-specific survival, 48%) tended to be worse than those with poorly differentiated squamous cell carcinoma (5-year disease-specific survival, 66%), but the difference did not reach statistical significance (P = 0.132). Clinical outcome of the small-cell carcinoma (all p16-positive) patients was also significantly shorter than that of p16-positive (P = 0.023) as well as that of p16-negative high-grade squamous cell carcinoma patients (P < 0.001) (Fig. 4). No significant difference was detected in clinical outcome between the patients with p16-positive and negative high-grade squamous cell carcinoma (P = 0.687, Fig. 4).
Clinical outcome of the patients. Disease-specific survival of the esophageal small-cell carcinoma patients was significantly worse than that of high-grade squamous cell carcinoma patients (P < 0.001). No significant difference was observed between the p16-positive and p16-negative high-grade squamous cell carcinoma patients (P = 0.687). Outcome of the small-cell carcinoma patients was also significantly shorter than that of the p16-positive and p16-negative groups of high-grade squamous cell carcinoma, respectively (P = 0.023 and P < 0.001, respectively)
Discussion
Esophageal small-cell carcinomas demonstrated consistent overexpression of p16 protein. However, p16 expression was not an exclusive event observed in small-cell carcinoma, also observed in 9% (7/82 cases) of high-grade squamous cell carcinomas. The p16 overexpression was associated with loss of Rb1 protein but not with HPV infection in both esophageal small-cell and high-grade squamous cell carcinomas.
p16 gene, also known as CDKN2A, is frequently inactivated in human malignant neoplasms [9, 10]. The results of a whole-exome sequencing analysis revealed that CDKN2A was inactivated in 49–76% of esophageal squamous cell carcinoma and is considered as one of the key events in cancer development [38,39,40]. CDKN2A was inactivated mostly by deletion (44–72%), occasionally by mutation (2–8%). CDKN2A DNA hypermethylation could also contribute to its inactivation, and the aberrant methylation of 5′ CpG islands of the CDKN2A promoter region leads to repression of its gene transcription [38]. In our present study, we focused on poorly differentiated and basaloid features of squamous cell carcinomas and the results revealed the frequency of p16 protein loss (91%). Of particular interest, Salam et al. reported an association of p16 overexpression with morphological differentiation of the tumor, i.e., loss of p16 expression was more frequently detected in poorly differentiated than in well- and moderately differentiated squamous cell carcinomas [44]. In addition, the status of p16 promoter methylation was also reported to be associated with histological differentiation, detected in up to 82% of poorly differentiated squamous cell carcinomas [44]. Although the frequency of inactivation of p16 in basaloid squamous cell carcinomas has not been fully studied at this juncture, an inactivation of the p16 gene might correlate with high-grade neoplasms. Whether p16 immunoreactivity can serve as a predictive marker for genetic or epigenetic inactivation of CDKN2A gene is controversial [41, 42], and further investigations are required for clarification at this juncture [41,42,43].
Strong nuclear and cytoplasmic expression of p16 observed in continuous areas of the epithelium, called “block” positivity, is typically observed in HPV-associated neoplasms. We have observed this staining pattern in all the small-cell carcinomas included in this study. The p16 overexpression is considered as positive feedback by deregulation of RB1 [9, 12]. An inactivation of RB1 was reported to play a pivotal role in carcinogenesis of neuroendocrine carcinomas, and Rb1 protein loss was proposed to be a characteristic in gastroenteropancreatic and pulmonary neuroendocrine carcinomas [6, 14]. In small-cell lung cancer, the mechanism has been shown that at least one allele of RB1 was affected by different genomic alterations (i.e., hemizygous deletion, loss of heterozygosity, or mutation, including rearrangements) [45]. As small-cell carcinomas of the esophagus and lung greatly share similar biological characteristics, it can be anticipated that these genetic alterations might contribute to the pathogenesis of esophageal small-cell carcinomas [6]. Fujimasa et al. reported that p16 overexpression and Rb1 protein loss were highly specific findings in esophageal small-cell carcinomas and their immunoreactivity could serve as a potentially useful diagnostic marker [12]. In contrast, the p16 expression pattern observed in high-grade squamous cell carcinomas was mostly focal or with a single-cell positivity. Interestingly, 5 out of 6 cases of high-grade squamous cell carcinomas with focal or a single-cell p16 expression also showed loss of Rb1 expression, which might suggest an incomplete activation of p16 protein despite the same p16/Rb1 signaling activation status as observed in small-cell carcinomas.
p16 overexpression in conventional squamous cell carcinoma of the esophagus was reported to be associated with better clinical outcome of the patients [28, 29]. However, in this study, p16 positivity in high-grade squamous cell carcinoma was not associated with tumor size, TNM stage, and patients’ clinical outcome. In the p16-positive high-grade squamous cell carcinoma group, only 4 cases presented the same expression pattern (p16 overexpression/Rb1 protein loss) as that of small-cell carcinomas. Interestingly, the p16 overexpression induced by dysregulation of RB1 appeared to have an association with tumor aggressiveness in high-grade squamous cell carcinoma. However, due to the limited number of cases available in our present study, further investigations are required for clarification. In addition, considering the high frequency of p16 loss in poorly differentiated squamous cell carcinomas, p16 status is by no means a favorable predictive factor of the patients. The patients with small-cell carcinoma, all of whom expressed p16, had significantly worse survival than those with p16-positive high-grade squamous cell carcinoma despite their common p16/Rb1 signaling activation status. This suggests that the p16/Rb1 signaling might not be a single regulatory mechanism that is associated with the aggressive behavior of the small-cell carcinomas. Due to the limited number of cases, further studies are needed to clarify the prognostic impact of p16 expression in highly malignant esophageal carcinomas. Nevertheless, the results of our present study confirmed the fact that poorly differentiated/basaloid squamous cell carcinomas must be differentiated from esophageal small-cell carcinomas by means of neuroendocrine markers (synaptophysin/chromogranin A) and a squamous-basal marker (e.g., p40).
Diffuse and marked p16 expression usually indicates the presence of HPV infection in squamous cell carcinoma of the uterine cervix, pharynx, and esophagus [15, 16, 28]. Cao et al. reported that the presence of HPV was significantly correlated with p16 immunoreactivity in esophageal squamous cell carcinoma and suggested the p16 immunoreactivity as a good marker for HPV infection [28]. However, p16 can be induced by dysregulation of the RB1 pathway without HPV infection. Indeed, in our present study, HPV infection was detected in none of the p16-positive small-cell and high-grade squamous cell carcinomas. In addition, 5–29% of esophageal squamous cell carcinomas were reported to present p16 expression without association with HPV infection [26, 28, 46, 47]. Based on the results above, immunohistochemical p16 positivity is not indicative of the presence of HPV infection in these highly malignant neoplasms. The reported incidence of HPV infection varies among geographic regions, detection methods, or primary sites. Therefore, further investigations are required for the detection of HPV infection in esophageal neoplasms in larger-scale studies [25,26,27,28, 46, 48].
It is also important to note the following limitations in this study. First, PCR to identify HPV genotypes was performed only in limited samples. Second, preoperative treatment might have affected the detection of HPV. Preoperative therapy was performed in some cases because it is the current standard treatment strategy for patients with localized and advanced squamous cell carcinoma of the esophagus in Japan [49]. Third, detection methods of HPV infection in the esophageal tissue have not been fully established. PCR is so far considered to be more reliable than in situ hybridization or immunohistochemistry [48]. These limitations are very hard to avoid in studying with relatively small scale of cases. Nevertheless, we believe despite all above-mentioned limitations, much can be learned from our data.
In summary, p16 overexpression is a constant, but not an exclusive finding in esophageal small-cell carcinomas and could be detected in poorly differentiated and basaloid squamous cell carcinomas albeit with less frequency. p16 overexpression in these highly malignant esophageal neoplasms is considered to represent the sequel of intracellular dysregulation of the RB1 signaling pathway and not HPV infection. The clinical outcome of esophageal small-cell carcinoma patients is significantly worse than that of p16-positive high-grade squamous cell carcinoma patients despite the common p16/Rb1 signaling activation status; however, this has to be further confirmed in a study with a higher number of the relevant neoplasms. Small-cell carcinomas and high-grade squamous cell carcinomas must be, due to the different clinical features, differentiated by neuroendocrine markers (synaptophysin/chromogranin A) and a squamous-basal marker (e.g., p40) expression. In addition, p16 overexpression does not indicate favorable clinical outcome in squamous cell carcinomas with poorly differentiated and basaloid morphology of the esophagus.
References
Tachimori Y, Ozawa S, Numasaki H, Ishihara R, Matsubara H, Muro K et al (2019) Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus 16:221–245
Abnet CC, Arnold M, Wei WQ (2018) Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154:360–373
Ishida H, Kasajima A, Onodera Y, Konno T, Maruyama S, Okamoto H, Sato C, Heishi T, Sakurai T, Taniyama Y, Takahashi M, Fujishima F, Jingu K, Ishioka C, Sasano H, Kamei T (2019) A comparative analysis of clinicopathological factors between esophageal small cell and basaloid squamous cell carcinoma. Medicine (Baltimore) 98:e14363
Imamhasan A, Mitomi H, Saito T, Hayashi T, Takahashi M, Kajiyama Y, Yao T (2012) Immunohistochemical and oncogenetic analyses of the esophageal basaloid squamous cell carcinoma in comparison with conventional squamous cell carcinomas. Hum Pathol 43:2012–2023
Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Li H, Liu DT, Chen YP (2012) Basaloid squamous cell carcinoma of the esophagus. J Cancer Res Clin Oncol 138:1165–1171
Ishida H, Kasajima A, Kamei T, Miura T, Oka N, Yazdani S, Ozawa Y, Fujishima F, Sakurada A, Nakamura Y, Tanaka Y, Kurosumi M, Ishikawa Y, Okada Y, Ohuchi N, Sasano H (2017) SOX2 and Rb1 in esophageal small-cell carcinoma: their possible involvement in pathogenesis. Mod Pathol 30:660–671
Feng JF, Huang Y, Zhao Q, Chen QX (2013) Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. The Scientific World Journal 2013:504365
Zhu Y, Qiu B, Liu H, Li Q, Xiao W, Hu Y, Liu M (2014) Primary small cell carcinoma of the esophagus: review of 64 cases from a single institution. Dis Esophagus 27:152–158
Romagosa C, Simonetti S, Lopez-Vicente L, Mazo A, Lleonart ME, Castellvi J et al (2011) p16 (Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 30:2087–2097
Roussel MF (1999) The INK4 family of cell cycle inhibitors in cancer. Oncogene 18:5311–5317
Serra S, Chetty R (2018) p16. J Clin Pathol 71:853–858
Fujimasa K, Ohike N, Norose T, Isobe T, Kikuchi K, Otsuka K et al (2019) Frequent and specific involvement of changes of the p16-RB pathway in esophageal neuroendocrine carcinoma. Anticancer Res 39:1927–1934
Dosaka-Akita H, Cagle PT, Hiroumi H, Fujita M, Yamashita M, Sharma A, Kawakami Y, Benedict WF (2000) Differential retinoblastoma and p16(INK4A) protein expression in neuroendocrine tumors of the lung. Cancer 88:550–556
Girardi DM, Silva ACB, Rego JFM, Coudry RA, Riechelmann RP (2017) Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: a systematic review. Cancer Treat Rev 56:28–35
Escobar N, Plugge E (2020) Prevalence of human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer in imprisoned women worldwide: a systematic review and meta-analysis. J Epidemiol Community Health 74:95–102
Devins KM, Tetzlaff MT, Baloch Z, LiVolsi VA (2019) The evolving landscape of HPV-related Neoplasia in the head and neck. Hum Pathol 94:29–39
Narisawa-Saito M, Kiyono T (2007) Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci 98:1505–1511
Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B (2010) Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope 120:1756–1772
Thompson LDR, Burchette R, Iganej S, Bhattasali O (2019) Oropharyngeal squamous cell carcinoma in 390 patients: analysis of clinical and histological criteria which significantly impact outcome. Head Neck Pathol. https://doi.org/10.1007/s12105-019-01096-0
Sathasivam HP, Santambrogio A, Andoniadou CL, Robinson M, Thavaraj S (2018) Prognostic utility of HPV specific testing in addition to p16 immunohistochemistry in oropharyngeal squamous cell carcinoma. Ann Oncol 29:2144–2145
Kawakami H, Okamoto I, Terao K, Sakai K, Suzuki M, Ueda S, Tanaka K, Kuwata K, Morita Y, Ono K, Nishio K, Nishimura Y, Doi K, Nakagawa K (2013) Human papillomavirus DNA and p16 expression in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer Med 2:933–941
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Ragin CC, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 121:1813–1820
Brierley JD, Gospodarowicz MK, Wittekind C (2017) Oesophagus and oesophagogastric junction. In: Union for International Cancer Control (ed) TNM classification of malignant tumours, 8th edn. John Wiley & Sons, Ltd, NewYork, pp 57–62
Bas Y, Aker FV, Gonultas A, Akdeniz R, Turgal E, Cikrikcioglu MA (2019) Effect of high-risk human papillomavirus in esophageal squamous cell carcinoma in Somalian and Turkish cases. Pathog Dis 77. https://doi.org/10.1093/femspd/ftz047
Pastrez PRA, Mariano VS, da Costa AM, Silva EM, Scapulatempo-Neto C, Guimaraes DP et al (2017) The relation of HPV infection and expression of p53 and p16 proteins in esophageal squamous cells carcinoma. J Cancer 8:1062–1070
Turkay DO, Vural C, Sayan M, Gurbuz Y (2016) Detection of human papillomavirus in esophageal and gastroesophageal junction tumors: a retrospective study by real-time polymerase chain reaction in an institutional experience from Turkey and review of literature. Pathol Res Pract 212:77–82
Cao F, Zhang W, Zhang F, Han H, Xu J, Cheng Y (2014) Prognostic significance of high-risk human papillomavirus and p16(INK4A) in patients with esophageal squamous cell carcinoma. Int J Clin Exp Med 7:3430–3438
Fujiwara S, Noguchi T, Takeno S, Kimura Y, Fumoto S, Kawahara K (2008) Hypermethylation of p16 gene promoter correlates with loss of p16 expression that results in poorer prognosis in esophageal squamous cell carcinomas. Dis Esophagus 21:125–131
Wang WL, Wang YC, Lee CT, Chang CY, Lo JL, Kuo YH, Hsu YC, Mo LR (2015) The impact of human papillomavirus infection on the survival and treatment response of patients with esophageal cancers. J Dig Dis 16:256–263
Odze RD, Lam AK, Ochiai A, Washington MK (2019) Tumours of the oesophagus. In: the WHO Classification of Timours Editorial Board (ed) Digestive system tumours WHO classification of tumours, 5th edn. International Agency for Research on Cancer, Lyon, pp 23–58
Schlederer M, Mueller KM, Haybaeck J, Heider S, Huttary N, Rosner M, Hengstschläger M, Moriggl R, Dolznig H, Kenner L (2014) Reliable quantification of protein expression and cellular localization in histological sections. PLoS One 9:e100822
Kasajima A, Pavel M, Darb-Esfahani S, Noske A, Stenzinger A, Sasano H, Dietel M, Denkert C, Rocken C, Wiedenmann B, Weichert W (2011) mTOR expression and activity patterns in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 18:181–192
Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, Agaimy A, Sipos B, Zamboni G, Weichert W, Esposito I, Pfarr N, Klöppel G (2017) Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol 30:587–598
Press OA, Zhang W, Gordon MA, Yang D, Lurje G, Iqbal S, el-Khoueiry A, Lenz HJ (2008) Gender-related survival differences associated with EGFR polymorphisms in metastatic colon cancer. Cancer Res 68:3037–3042
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Fujinaga Y, Shimada M, Okazawa K, Fukushima M, Kato I, Fujinaga K (1991) Simultaneous detection and typing of genital human papillomavirus DNA using the polymerase chain reaction. J Gen Virol 72:1039–1044
Bass AJ, Laird PW, Shmulevich I et al (2017) Integrated genomic characterization of oesophageal carcinoma. Nature 541:169–175
Sawada G, Niida A, Uchi R, Hirata H, Shimamura T, Suzuki Y, Shiraishi Y, Chiba K, Imoto S, Takahashi Y, Iwaya T, Sudo T, Hayashi T, Takai H, Kawasaki Y, Matsukawa T, Eguchi H, Sugimachi K, Tanaka F, Suzuki H, Yamamoto K, Ishii H, Shimizu M, Yamazaki H, Yamazaki M, Tachimori Y, Kajiyama Y, Natsugoe S, Fujita H, Mafune K, Tanaka Y, Kelsell DP, Scott CA, Tsuji S, Yachida S, Shibata T, Sugano S, Doki Y, Akiyama T, Aburatani H, Ogawa S, Miyano S, Mori M, Mimori K (2016) Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology 150:1171–1182
Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, Ma X, Liu L, Zhao Z, Huang X, Fan J, Dong L, Chen G, Ma L, Yang J, Chen L, He M, Li M, Zhuang X, Huang K, Qiu K, Yin G, Guo G, Feng Q, Chen P, Wu Z, Wu J, Ma L, Zhao J, Luo L, Fu M, Xu B, Chen B, Li Y, Tong T, Wang M, Liu Z, Lin D, Zhang X, Yang H, Wang J, Zhan Q (2014) Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509:91–95
Lim AM, Do H, Young RJ, Wong SQ, Angel C, Collins M, Takano EA, Corry J, Wiesenfeld D, Kleid S, Sigston E, Lyons B, Fox SB, Rischin D, Dobrovic A, Solomon B (2014) Differential mechanisms of CDKN2A (p16) alteration in oral tongue squamous cell carcinomas and correlation with patient outcome. Int J Cancer 135:887–895
Sterlacci W, Tzankov A, Veits L, Zelger B, Bihl MP, Foerster A, Augustin F, Fiegl M, Savic S (2011) A comprehensive analysis of p16 expression, gene status, and promoter hypermethylation in surgically resected non-small cell lung carcinomas. J Thorac Oncol 6:1649–1657
Tokugawa T, Sugihara H, Tani T, Hattori T (2002) Modes of silencing of p16 in development of esophageal squamous cell carcinoma. Cancer Res 62:4938–4944
Salam I, Hussain S, Mir MM, Dar NA, Abdullah S, Siddiqi MA, Lone RA, Zargar SA, Sharma S, Hedau S, Basir SF, Bharti AC, Das BC (2009) Aberrant promoter methylation and reduced expression of p16 gene in esophageal squamous cell carcinoma from Kashmir valley: a high-risk area. Mol Cell Biochem 332:51–58
Peifer M, Fernandez-Cuesta L, Sos ML, George J, Seidel D, Kasper LH et al (2012) Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 44:1104–1110
Lofdahl HE, Du J, Nasman A, Andersson E, Rubio CA, Lu Y et al (2012) Prevalence of human papillomavirus (HPV) in oesophageal squamous cell carcinoma in relation to anatomical site of the tumour. PLoS One 7:e46538
Ding GC, Ren JL, Chang FB, Li JL, Yuan L, Song X, Zhou SL, Guo T, Fan ZM, Zeng Y, Wang LD (2010) Human papillomavirus DNA and P16(INK4A) expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol 16:5901–5906
Zhou XB, Guo M, Quan LP, Zhang W, Lu ZM, Wang QH, Ke Y, Xu NZ (2003) Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World J Gastroenterol 9:1170–1173
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74
Acknowledgments
The authors would like to acknowledge Dr. Yoichi Tanaka and Dr. Masafumi Kurosumi (Saitama Cancer Center, Saitama, Japan), Dr. Masahiro Chin and Dr. Akiko Nishida (Nihonkai General Hospital, Yamagata, Japan), Dr. Go Miyata and Dr. Tsutomu Sakuma (Iwate Prefectural Central Hospital, Iwate, Japan), Dr. Shunsuke Shibuya and Dr. Kazuyuki Ishida (Iwate Prefectural Isawa Hospital, Iwate, Japan), and Dr. Kenichi Yokota (Kesennuma City Hospital, Miyagi, Japan) for providing tissue samples and clinical data. We also thank Ms. Kazue Ise, Ms. Erina Iwabuchi, Ms. Yayoi Aoyama, Mr. Katsuhiko Ono, and Ms. Yasuko Furukawa (Tohoku University, Sendai, Japan); Dr. Naomi Oka, Dr. Junko Sakurada, and Dr. Hiroyoshi Suzuki (National Hospital Organization Sendai Medical Center, Sendai, Japan); and Dr. Ichiro Abe and Dr. Makiko Abe (Griffith University, Gold Coast, Australia) for their excellent technical and statistical support.
Funding
Open Access funding provided by Projekt DEAL. This work was supported in part by the Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (JSPS KAKENHI Grant Number JP26460413) and funding provided by Alexander von Humboldt foundation (to A.K.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Hirotaka Ishida, Fumiyoshi Fujishima, Ryujiro Akaishi, Shunsuke Ueki, Yuto Yamazaki, and Xin Gao. Immunohistochemical staining was performed by Hirotaka Ishida, Atsuko Kasajima, Fumiyoshi Fujishima, Ryujiro Akaishi, and Shunsuke Ueki. Hirotaka Ishida, Atsuko Kasajima, Fumiyoshi Fujishima, and Hironobu Sasano evaluated the results of immunohistochemistry. Polymerase chain reaction was conducted by Hirotaka Ishida, Yuto Yamazaki, and Yoshiaki Onodera. Data collection was performed by Hirotaka Ishida, Ryujiro Akaishi, Shunsuke Ueki, Hiroshi Okamoto, Yusuke Taniyama, and Takashi Kamei. The data was analyzed by Hirotaka Ishida, Atsuko Kasajima, and Yoshiaki Onodera. Hironobu Sasano and Takashi Kamei supervised the project. The first draft of the manuscript was written by Hirotaka Ishida and Atsuko Kasajima, and all authors commented on the manuscript. Manuscript editing was performed by all authors. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The study protocol was approved by the ethics committee or institutional review board of each participating institution (Accession number of Tohoku University Hospital 2018–1–151).
Consent to participate/for publication
Informed consents were obtained from all patients for participating and publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
Summary of evaluation methods for immunohistochemistry used in this study (XLSX 12 kb)
Supplementary Table 2
Clinicopathological characteristics of 97 patients with esophageal small cell and high-grade squamous cell carcinomas (XLSX 12 kb)
Supplementary Table 3
Comparison of clinicopathological features of p16 positive and negative high-grade squamous cell carcinomas (XLSX 12 kb)
Supplementary Figure 1
Representative illustrations of immunohistochemistry for p16 (a) and Rb1 (b) in basaloid squamous cell carcinoma. p16 expression in non-neoplastic squamous epithelium and several stromal cells (arrowheads, a) served as a positive internal control of p16, whereas carcinoma cells showed negativity for p16 (arrows, a). Nuclear Rb1 immunoreactivity was observed in more than 50% of carcinoma cells with variable intensity (b). Rb1 is also expressed in some stromal cells and non-neoplastic epithelium (b). Histopathological image of cervical intraepithelial neoplasia (c, hematoxylin and eosin staining) and immunohistochemical staining for p16 in the corresponding area (d). Strong nuclear immunoreactivity of p16 was observed in neoplastic cells, whereas majority of non-neoplastic epithelial cells were negative for p16 (d) (PNG 5082 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishida, H., Kasajima, A., Fujishima, F. et al. p16 in highly malignant esophageal carcinomas: the correlation with clinicopathological factors and human papillomavirus infection. Virchows Arch 478, 219–229 (2021). https://doi.org/10.1007/s00428-020-02865-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02865-x