Abstract
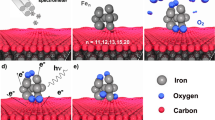
Nonprecious metal catalysts are known of significance for electrochemical N2 reduction reaction (NRR) of which the mechanism has been illustrated by ongoing investigations of single atom catalysis. However, it remains challenging to fully understand the size-dependent synergistic effect of active sites inherited in substantial nanocatalysts. In this work, four types of small iron clusters Fen (n = 1–4) supported on nitrogen-doped graphene sheets are constructed to figure out the size dependence and synergistic effect of active sites for NRR catalytic activities. It is revealed that Fe3 and Fe4 clusters on N4G supports exhibit higher NRR activity than single-iron atom and iron dimer clusters, showing lowered limiting potential and restricted hydrogen evolution reaction (HER) which is a competitive reaction channel. In particular, the Fe4-N4G displays outstanding NRR performance for “side-on” adsorption of N2 with a small limiting potential (−0.45 V). Besides the specific structure and strong interface interaction within the Fe4-N4G itself, the high NRR activity is associated with the unique bonding/antibonding orbital interactions of N-N and N-Fe for the adsorptive N2 and NNH intermediates, as well as relatively large charge transfer between N2 and the cluster Fe4-N4G.

Similar content being viewed by others
References
Guo, C. X.; Ran, J. R.; Vasileff, A.; Qiao, S. Z. Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci.2018, 11, 45–56.
Ashida, Y.; Arashiba, K.; Nakajima, K.; Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature2019, 568, 536–540.
Nagaoka, K.; Eboshi, T.; Takeishi, Y.; Tasaki, R.; Honda, K.; Imamura, K.; Sato, K. Carbon-free H2 production from ammonia triggered at room temperature with an acidic RuO2/γ-Al2O3 catalyst. Sci. Adv.2017, 3, e1602747.
Wang, L.; Xia, M. K.; Wang, H.; Huang, K. F.; Qian, C. X.; Maravelias, C. T.; Ozin, G. A. Greening ammonia toward the solar ammonia refinery. Joule2018, 2, 1055–1074.
Bhutto, S. M.; Holland, P. L. Dinitrogen activation and functionalization using β-diketiminate iron complexes. Eur. J. Inorg. Chem.2019, 2019, 1861–1869.
Gong, Y. T.; Wu, J. Z.; Kitano, M.; Wang, J. J.; Ye, T. N.; Li, J.; Kobayashi, Y.; Kishida, K.; Abe, H.; Niwa, Y. et al. Ternary intermetallic LaCoSi as a catalyst for N2 activation. Nat. Catal.2018, 1, 178–185.
Kobayashi, Y.; Tang, Y.; Kageyama, T.; Yamashita, H.; Masuda, N.; Hosokawa, S.; Kageyama, H. Titanium-based hydrides as heterogeneous catalysts for ammonia synthesis. J. Am. Chem. Soc.2017, 139, 18240–18246.
Jia, H. L.; Du, A. X.; Zhang, H.; Yang, J. H.; Jiang, R. B.; Wang, J. F.; Zhang, C. Y. Site-selective growth of crystalline ceria with oxygen vacancies on gold nanocrystals for near-infrared nitrogen photofixation. J. Am. Chem. Soc.2019, 141, 5083–5086.
Shi, M. M.; Bao, D.; Wulan, B. R.; Li, Y. H.; Zhang, Y. F.; Yan, J. M.; Jiang, Q. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater.2017, 29, 1606550.
Cui, X. Y.; Tang, C.; Zhang, Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater.2018, 8, 1800369.
Xue, X. L.; Chen, R. P.; Yan, C. Z.; Zhao, P. Y.; Hu, Y.; Zhang, W. J.; Yang, S. Y.; Jin, Z. Review on photocatalytic and electrocatalytic artificial nitrogen fixation for ammonia synthesis at mild conditions: Advances, challenges and perspectives. Nano Res.2019, 12, 1229–1249.
Wang, F.; Ma, J. Z.; He, G. Z.; Chen, M.; Zhang, C. B.; He, H. Nanosize effect of Al2O3 in Ag/Al2O3 catalyst for the selective catalytic oxidation of ammonia. ACS Catal.2018, 8, 2670–2682.
Tsuji, Y.; Ogasawara, K.; Kitano, M.; Kishida, K.; Abe, H.; Niwa, Y.; Yokoyama, T.; Hara, M.; Hosono, H. Control of nitrogen activation ability by Co-Mo bimetallic nanoparticle catalysts prepared via sodium naphthalenide-reduction. J. Catal.2018, 364, 31–39.
Wang, A. Q.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem.2018, 2, 65–81.
Zheng, S. S.; Li, S. N.; Mei, Z. W.; Hu, Z. X.; Chu, M. H.; Liu, J. H.; Chen, X.; Pan, F. Electrochemical nitrogen reduction reaction performance of single-boron catalysts tuned by MXene substrates. J. Phys. Chem. Lett.2019, 10, 6984–6989.
Zang, W. J.; Yang, T.; Zou, H. Y.; Xi, S. B.; Zhang, H.; Liu, X. M.; Kou, Z. K.; Du, Y. H.; Feng, Y. P.; Shen, L. et al. Copper single atoms anchored in porous nitrogen-doped carbon as efficient pH-universal catalysts for the nitrogen reduction reaction. ACS Catal.2019, 9, 10166–10173.
He, C.; Wu, Z. Y.; Zhao, L.; Ming, M.; Zhang, Y.; Yi, Y. P.; Hu, J. S. Identification of FeN4 as an efficient active site for electrochemical N2 reduction. ACS Catal.2019, 9, 7311–7317.
Li, X. F.; Li, Q. K.; Cheng, J.; Liu, L. L.; Yan, Q.; Wu, Y. C.; Zhang, X. H.; Wang, Z. Y.; Qiu, Q.; Luo, Y. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J. Am. Chem. Soc.2016, 138, 8706–8709.
Wang, Y.; Cui, X. Q.; Zhao, J. X.; Jia, G. R.; Gu, L.; Zhang, Q. H.; Meng, L. K.; Shi, Z.; Zheng, L. R.; Wang, C. Y. et al. Rational design of Fe-N/C hybrid for enhanced nitrogen reduction electrocatalysis under ambient conditions in aqueous solution. ACS Catal.2019, 9, 336–344.
Lü, F.; Zhao, S. Z.; Guo, R. J.; He, J.; Peng, X. Y.; Bao, H. H.; Fu, J. T.; Han, L. L.; Qi, G. C.; Luo, J. et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media. Nano Energy2019, 61, 420–427.
Guo, X. Y.; Huang, S. P. Tunin g nitrogen reduction reaction activity via controllable Fe magnetic moment: A computational study of single Fe atom supported on defective graphene. Electrochim. Acta2018, 284, 392–399.
Wei, Z. X.; Zhang, Y. F.; Wang, S. Y.; Wang, C. Y.; Ma, J. M. Fe-doped phosphorene for the nitrogen reduction reaction. J. Mater. Chem. A2018, 6, 13790–13796.
Choi, C.; Back, S.; Kim, N. Y.; Lim, J.; Kim, Y. H.; Jung, Y. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: A computational guideline. ACS Catal.2018, 8, 7517–7525.
Georgakilas, V.; Tiwari, J. N.; Kemp, K. C.; Perman, J. A.; Bourlinos, A. B.; Kim, K. S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev.2016, 116, 5464–5519.
Tyo, E. C.; Vajda, S. Catalysis by clusters with precise numbers of atoms. Nat. Nanotechnol.2015, 10, 577–588.
Cui, C. N.; Luo, Z. X.; Yao, J. N. Enhanced catalysis of Pt3 clusters supported on graphene for N-H bond dissociation. CCS Chem.2019, 1, 215–225.
An, J. H.; Wang, Y. H.; Lu, J. M.; Zhang, J.; Zhang, Z. X.; Xu, S. T.; Liu, X. Y.; Zhang, T.; Gocyla, M.; Heggen, M. et al. Acid-promoter-free ethylene methoxycarbonylation over Ru-clusters/ceria: The catalysis of interfacial lewis acid-base pair. J. Am. Chem. Soc.2018, 140, 4172–4181.
Ren, Y.; Yang, Y.; Zhao, Y. X.; He, S. G. Size-dependent reactivity of rhodium cluster anions toward methane. J. Phys. Chem. C2019, 123, 17035–17042.
Yang, B.; Liu, C.; Halder, A.; Tyo, E. C.; Martinson, A. B. F.; Seifer, S.; Zapol, P.; Curtiss, L. A.; Vajda, S. Copper cluster size effect in methanol synthesis from CO2. J. Phys. Chem. C2017, 121, 10406–10412.
Kong, J. M.; Lim, A.; Yoon, C.; Jang, J. H.; Ham, H. C.; Han, J.; Nam, S.; Kim, D.; Sung, Y. E.; Choi, J. et al. Electrochemical synthesis of NH3 at low temperature and atmospheric pressure using a γ-Fe2O3 catalyst. ACS Sustainable Chem. Eng.2017, 5, 10986–10995.
Manjunatha, R.; Karajić, A.; Goldstein, V.; Schechter, A. Electrochemical ammonia generation directly from nitrogen and air using an iron-oxide/titania-based catalyst at ambient conditions. ACS Appl. Mater. Interfaces2019, 11, 7981–7989.
Xia, L.; Li, B. H.; Zhang, Y.; Zhang, R.; Ji, L.; Chen, H. Y.; Cui, G. W.; Zheng, H. G.; Sun, X. P.; Xie, F. Y. et al. Cr2O3 nanoparticle-reduced graphene oxide hybrid: A highly active electrocatalyst for N2 reduction at ambient conditions. Inorg. Chem.2019, 58, 2257–2260.
Chen, G. F.; Ren, S. Y.; Zhang, L. L.; Cheng, H.; Luo, Y. R.; Zhu, K. H.; Ding, L. X.; Wang, H. H. Advances in electrocatalytic N2 reduction-strategies to tackle the selectivity challenge. Small Methods2019, 3, 1800337.
Li, S. J.; Bao, D.; Shi, M. M.; Wulan, B. R.; Yan, J. M.; Jiang, Q. Amorphizing of Au nanoparticles by CeOx-RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions}. Adv. Mater.2017, 29, 1700001.
Nazemi, M.; El-Sayed, M. A. Electrochemical synthesis of ammonia from N2 and H2O under ambient conditions using pore-size-controlled hollow gold nanocatalysts with tunable plasmonic properties. J. Phys. Chem. Lett.2018, 9, 5160–5166.
Chen, S. M.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D. S.; Centi, G. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst. Angew. Chem., Int. Ed.2017, 56, 2699–2703.
Yao, Y.; Zhu, S. Q.; Wang, H. J.; Li, H.; Shao, M. H. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc.2018, 140, 1496–1501.
Zhao, S. L.; Lu, X. Y.; Wang, L. Z.; Gale, J.; Amal, R. Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions. Adv. Mater.2019, 31, 1805367.
Tanaka, H.; Nishibayashi, Y.; Yoshizawa, K. Interplay between theory and experiment for ammonia synthesis catalyzed by transition metal complexes. Acc. Chem. Res.2016, 49, 987–995.
Licht, S.; Cui, B. C.; Wang, B. H.; Li, F. F.; Lau, J.; Liu, S. Z. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science2014, 345, 637–640.
Kosaka, F.; Nakamura, T.; Oikawa, A.; Otomo, J. Electrochemical acceleration of ammonia synthesis on Fe-based alkali-promoted electrocatalyst with proton conducting solid electrolyte. ACS Sustainable Chem. Eng.2017, 5, 10439–10446.
Hu, L.; Khaniya, A.; Wang, J.; Chen, G.; Kaden, W. E.; Feng, X. F. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe oxide catalyst. ACS Catal.2018, 8, 9312–9319.
Qian, J.; An, Q.; Fortunelli, A.; Nielsen, R. J.; Goddard III, W. A. Reaction mechanism and kinetics for ammonia synthesis on the Fe(111) surface. J. Am. Chem. Soc.2018, 140, 6288–6297.
Li, C.; Fu, Y. S.; Wu, Z.; Xia, J. W.; Wang, X. Sandwich-like reduced graphene oxide/yolk-shell-structured Fe@Fe3O4/carbonized paper as an efficient freestanding electrode for electrochemical synthesis of ammonia directly from H2O and nitrogen. Nanoscale2019, 11, 12997–13006.
Maheshwari, S.; Rostamikia, G.; Janik, M. J. Elementary kinetics of nitrogen electroreduction on Fe surfaces. J. Chem. Phys.2019, 150, 041708.
Mou, X. L.; Zhang, B. S.; Li, Y.; Yao, L. D.; Wei, X. J.; Su, D. S.; Shen, W. J. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem., Int. Ed.2012, 51, 2989–2993.
Zhang, L. F.; Zhao, W. H.; Zhang, W. H.; Chen, J.; Hu, Z. P. gt-C3N4 coordinated single atom as an efficient electrocatalyst for nitrogen reduction reaction. Nano Res.2019, 12, 1181–1186.
Yang, L. M.; Yi, G. P.; Hou, Y. N.; Cheng, H. Y.; Luo, X. B.; Pavlostathis, S. G.; Luo, S. L.; Wang, A. J. Building electrode with three-dimensional macroporous interface from biocompatible polypyrrole and conductive graphene nanosheets to achieve highly efficient microbial electrocatalysis. Biosens. Bioelectron.2019, 141, 111444.
Luo, Z. X.; Castleman, A. W. Jr.; Khanna, S. N. Reactivity of metal clusters. Chem. Rev.2016, 116, 14456–14492.
Luo, Z. X.; Castleman, A. W. Special and general superatoms. Acc. Chem. Res.2014, 47, 2931–2940.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B1996, 54, 11169–11186.
Wang, R. B.; Hellman, A. Hybrid functional study of the electro-oxidation of water on pristine and defective hematite (0001). J. Phys. Chem. C2019, 123, 2820–2827.
Rohling, R. Y.; Tranca, I. C.; Hensen, E. J. M.; Pidko, E. A. Correlations between density-based bond orders and orbital-based bond energies for chemical bonding analysis. J. Phys. Chem. C2019, 123, 2843–2854.
Maintz, S.; Deringer, V. L.; Tchougreeff, A. L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem.2016, 37, 1030–1035.
Maintz, S.; Deringer, V. L.; Tchougréeff, A. L.; Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem.2013, 34, 2557–2567.
Malko, D.; Kucernak, A.; Lopes, T. In situ electrochemical quantification of active sites in Fe-N/C non-precious metal catalysts. Nat. Commun.2016, 7, 13285.
Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B2004, 108, 17886–17892.
Cui, C. N.; Han, J. Y.; Zhu, X. L.; Liu, X.; Wang, H.; Mei, D. H.; Ge, Q. F. Promotional effect of surface hydroxyls on electrochemical reduction of CO2 over SnOx/Sn electrode. J. Catal.2016, 343, 257–265.
Psofogiannakis, G.; St-Amant, A.; Ternan, M. Methane oxidation mechanism on Pt(111): A cluster model DFT study. J. Phys. Chem. B2006, 110, 24593–24605.
Montoya, J. H.; Tsai, C.; Vojvodic, A.; Nørskov, J. K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem2015, 8, 2180–2186.
Liu, J. C.; Ma, X. L.; Li, Y.; Wang, Y. G.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun.2018, 9, 1610.
McWilliams, S. F.; Holland, P. L. Dinitrogen binding and cleavage by multinuclear iron complexes. Acc. Chem. Res.2015, 48, 2059–2065.
Hoffman, B. M.; Lukoyanov, D.; Yang, Z. Y.; Dean, D. R.; Seefeldt, L. C. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem. Rev.2014, 114, 4041–4062.
Liu, C. W.; Li, Q. Y.; Wu, C. Z.; Zhang, J.; Jin, Y. G.; MacFarlane, D. R.; Sun, C. H. Single-boron catalysts for nitrogen reduction reaction. J. Am. Chem. Soc.2019, 141, 2884–2888.
Lu, Z. Y.; Chen, G. X.; Siahrostami, S.; Chen, Z. H.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D. C.; Liu, Y. Y. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal.2018, 1, 156–162.
Liu, H. N.; Li, W.; Liu, F.; Pei, Z. Z.; Shi, J.; Wang, Z. J.; He, D. H.; Chen, Y. Homogeneous, heterogeneous, and biological catalysts for electrochemical N2 reduction toward NH3 under ambient conditions. ACS Catal.2019, 9, 5245–5267.
Van Der Ham, C. J. M.; Koper, M. T.; Hetterscheid, D. G. H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev.2014, 43, 5183–5191.
Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J. K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys.2012, 14, 1235–1245.
Wang, H. W.; Gu, X. K.; Zheng, X. S.; Pan, H. B.; Zhu, J. F.; Chen, S.; Cao, L. N.; Li, W. X.; Lu, J. L. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv.2019, 5, eaat6413.
Ma, X. L.; Liu, J. C.; Xiao, H.; Li, J. Surface single-cluster catalyst for N2-to-NH3 thermal conversion. J. Am. Chem. Soc.2018, 140, 46–49.
Ling, C. Y.; Bai, X. W.; Ouyang, Y. X.; Du, A. J.; Wang, J. L. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions. J. Phys. Chem. C2018, 122, 16842–16847.
Deringer, V. L.; Tchougreeff, A. L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A2011, 115, 5461–5466.
Dronskowski, R.; Bloechl, P. E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem.1993, 97, 8617–8624.
Hao, Y. C.; Guo, Y.; Chen, L. W.; Shu, M.; Wang, X. Y.; Bu, T. A.; Gao, W. Y.; Zhang, N.; Su, X.; Feng, X. et al. Promoting nitrogen electro-reduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal.2019, 2, 448–456.
Bickelhaupt, F. M.; Nagle, J. K.; Klemm, W. L. Role of s-p orbital mixing in the bonding and properties of second-period diatomic molecules. J. Phys. Chem. A2008, 112, 2437–2446.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21802146 and 21722308), CAS Key Research Project of Frontier Science (No. QYZDB-SSW-SLH024), and Frontier Cross Project of National Laboratory for Molecular Sciences (No. 051Z011BZ3).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2020_2847_MOESM1_ESM.pdf
Nitrogen reduction reaction on small iron clusters supported by N-doped graphene: A theoretical study of the atomically precise active-site mechanism
Rights and permissions
About this article
Cite this article
Cui, C., Zhang, H. & Luo, Z. Nitrogen reduction reaction on small iron clusters supported by N-doped graphene: A theoretical study of the atomically precise active-site mechanism. Nano Res. 13, 2280–2288 (2020). https://doi.org/10.1007/s12274-020-2847-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2847-0