Abstract
Purpose
Surgery with radiation therapy (RT) is more effective in treating spinal metastases, than RT alone. However, RT when administered in close proximity to surgery may predispose to wound complications. There exist limited guidelines on the optimal timing between RT and surgery. The purpose of this systematic review is to: (1) address whether pre-operative RT (preop-RT) and/or post-operative RT (postop-RT) is associated with wound complications and (2) define the safe interval between RT and surgery or vice versa.
Methods
PubMed, Embase and Scopus databases were systematically searched for articles dealing with spinal metastases, treated with surgery and RT, and discussing wound status.
Results
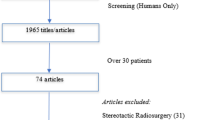
We obtained 2332 articles from all databases, and after applying exclusion criteria, removing duplicates and reading the full text, we identified 27 relevant articles. Fourteen additional articles were identified by hand-search, leading to a total of 41 articles. All 41 mentioned wound complications/healing. Sixteen articles discussed preop-RT, 8 postop-RT, 15 both, and 2 mentioned intraoperative-RT with additional pre/postop-RT. Twenty studies mentioned surgery-RT time interval; one concluded that wound complications were higher when RT-surgery interval was ≤ 7 days. Seven studies reported significant association between preop-RT and wound complications.
Conclusions
Evidence is insufficient to draw definitive conclusion about optimal RT-surgery interval. However, based on published literature and expert opinions, we conclude that an interval of 2 weeks, the minimum being 7 days, is optimum between RT-surgery or vice versa; this can be reduced further by postop-stereotactic body RT. If RT-surgery window is > 12 months, wound-complications rise. Postop-RT has fewer wound complications versus preop-RT.

Similar content being viewed by others
References
Jacobs WB, Perrin RG (2001) Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus 11(6):e10. https://doi.org/10.3171/foc.2001.11.6.11
Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, Melcher R, Tomita K, Global Spine Tumor Study G (2010) Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 19(2):215–222. https://doi.org/10.1007/s00586-009-1252-x
Kumar N, Vijayaraghavan G, Ravikumar N, Ding Y, Yin ML, Patel RS, Naresh N, Hey HWD, Lau L-L, Liu G (2019) Intraoperative neuromonitoring (IONM): is there a role in metastatic spine tumor surgery? Spine 44(4):E219–E224. https://doi.org/10.1097/BRS.0000000000002808
Kumar N, Malhotra R, Zaw AS, Maharajan K, Naresh N, Kumar A, Vellayappan B (2017) Evolution in treatment strategy for metastatic spine disease: presently evolving modalities. Eur J Surg Oncol 43(9):1784–1801. https://doi.org/10.1016/j.ejso.2017.05.006
Thomas KC, Nosyk B, Fisher CG, Dvorak M, Patchell RA, Regine WF, Loblaw A, Bansback N, Guh D, Sun H, Anis A (2006) Cost-effectiveness of surgery plus radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys 66(4):1212–1218. https://doi.org/10.1016/j.ijrobp.2006.06.021
Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF (2006) Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976) 31(24):2849–2856. https://doi.org/10.1097/01.brs.0000245838.37817.40
Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Mohiuddin M, Young B (2005) Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 366(9486):643–648. https://doi.org/10.1016/S0140-6736(05)66954-1
Tibbs MK (1997) Wound healing following radiation therapy: a review. Radiother Oncol 42(2):99–106. https://doi.org/10.1016/s0167-8140(96)01880-4
Arbeit JM, Hilaris BS, Brennan MF (1987) Wound complications in the multimodality treatment of extremity and superficial truncal sarcomas. J Clin Oncol 5(3):480–488. https://doi.org/10.1200/JCO.1987.5.3.480
Hill RP, Kaspler P, Griffin AM, O'Sullivan B, Catton C, Alasti H, Abbas A, Heydarian M, Ferguson P, Wunder JS, Bell RS (2007) Studies of the in vivo radiosensitivity of human skin fibroblasts. Radiother Oncol 84(1):75–83. https://doi.org/10.1016/j.radonc.2007.05.025
Gorodetsky R, McBride WH, Withers HR (1988) Assay of radiation effects in mouse skin as expressed in wound healing. Radiat Res 116(1):135–144. https://doi.org/10.2307/3577484
Itshayek E, Cohen JE, Yamada Y, Gokaslan Z, Polly DW, Rhines LD, Schmidt MH, Varga PP, Mahgarefteh S, Fraifeld S, Gerszten PC, Fisher CG (2014) Timing of stereotactic radiosurgery and surgery and wound healing in patients with spinal tumors: a systematic review and expert opinions. Neurol Res 36(6):510–523. https://doi.org/10.1179/1743132814Y.0000000380
Mustoe TA, Porras-Reyes BH (1993) Modulation of wound healing response in chronic irradiated tissues. Clin Plast Surg 20(3):465–472
O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B (2002) Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359(9325):2235–2241. https://doi.org/10.1016/S0140-6736(02)09292-9
Chadwick M, Vieten D, Pettitt E, Dixon A, Roe A (2006) Short course preoperative radiotherapy is the single most important risk factor for perineal wound complications after abdominoperineal excision of the rectum. Colorectal Dis 8(9):756–761. https://doi.org/10.1111/j.1463-1318.2006.01029.x
Vellayappan BA, Chao ST, Foote M, Guckenberger M, Redmond KJ, Chang EL, Mayr NA, Sahgal A, Lo SS (2018) The evolution and rise of stereotactic body radiotherapy (SBRT) for spinal metastases. Expert Rev Anticancer Ther 18(9):887–900. https://doi.org/10.1080/14737140.2018.1493381
Low DA, Mutic S (1998) A commercial IMRT treatment-planning dose-calculation algorithm. Int J Radiat Oncol Biol Phys 41(4):933–937. https://doi.org/10.1016/S0360-3016(98)00129-1
Ryu SI, Chang SD, Kim DH, Murphy MJ, Le QT, Martin DP, Adler JR Jr (2001) Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery 49(4):838–846. https://doi.org/10.1097/00006123-200110000-00011
Versteeg AL, Hes J, van der Velden JM, Eppinga W, Kasperts N, Verkooijen HM, van Vulpen M, Oner FC, Seravalli E, Verlaan JJ (2019) Sparing the surgical area with stereotactic body radiotherapy for combined treatment of spinal metastases: a treatment planning study. Acta Oncol 58(2):251–256. https://doi.org/10.1080/0284186X.2018.1539240
Kirkpatrick JP, van der Kogel AJ, Schultheiss TE (2010) Radiation dose and volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 76(3):S42–S49. https://doi.org/10.1016/j.ijrobp.2009.04.095
Sahgal A, Chang JH, Ma L, Marks LB, Milano MT, Medin P, Niemierko A, Soltys SG, Tome WA, Wong CS, Yorke E, Grimm J, Jackson A (2019) Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2019.09.038
Ghogawala Z, Mansfield FL, Borges LF (2001) Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine (Phila Pa 1976) 26(7):818–824. https://doi.org/10.1097/00007632-20010401000025
Versteeg AL, van der Velden JM, Hes J, Eppinga W, Kasperts N, Verkooijen HM, Oner FC, Seravalli E, Verlaan JJ (2018) Stereotactic radiotherapy followed by surgical stabilization within 24 h for unstable spinal metastases; A Stage I/IIa study according to the IDEAL framework. Front Oncol 8(1):626. https://doi.org/10.3389/fonc.2018.00626
Szczerba P (2019) Complications after surgical treatment of spinal metastases. Ortop Traumatol Rehabil 21(1):23–31. https://doi.org/10.5604/01.3001.0013.1077
Sundaresan N, Rothman A, Manhart K, Kelliher K (2002) Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 27(16):1802–1806. https://doi.org/10.1097/00007632-200208150-00021
Sugita S, Hozumi T, Yamakawa K, Goto T, Kondo T (2016) Risk factors for surgical site infection after posterior fixation surgery and intraoperative radiotherapy for spinal metastases. Eur Spine J 25(4):1034–1038. https://doi.org/10.1007/s00586-015-4116-6
Pascal-Moussellard H, Broc G, Pointillart V, Simeon F, Vital JM, Senegas J (1998) Complications of vertebral metastasis surgery. Eur Spine J 7(6):438–444. https://doi.org/10.1007/s005860050105
Omeis IA, Dhir M, Sciubba DM, Gottfried ON, McGirt MJ, Attenello FJ, Wolinsky JP, Gokaslan ZL (2011) Postoperative surgical site infections in patients undergoing spinal tumor surgery: incidence and risk factors. Spine (Phila Pa 1976) 36(17):1410–1419. https://doi.org/10.1097/BRS.0b013e3181f48fa9
Demura S, Kawahara N, Murakami H, Nambu K, Kato S, Yoshioka K, Okayama T, Tomita K (2009) Surgical site infection in spinal metastasis: risk factors and countermeasures. Spine (Phila Pa 1976) 34(6):635–639. https://doi.org/10.1097/BRS.0b013e31819712ca
Holman PJ, Suki D, McCutcheon I, Wolinsky JP, Rhines LD, Gokaslan ZL (2005) Surgical management of metastatic disease of the lumbar spine: experience with 139 patients. J Neurosurg Spine 2(5):550–563. https://doi.org/10.3171/spi.2005.2.5.0550
Keam J, Bilsky MH, Laufer I, Shi W, Zhang Z, Tam M, Zatcky J, Lovelock DM, Yamada Y (2014) No association between excessive wound complications and preoperative high-dose, hypofractionated, image-guided radiation therapy for spine metastasis. J Neurosurg Spine 20(4):411–420. https://doi.org/10.3171/2013.12.SPINE12811
Kumar S, van Popta D, Rodrigues-Pinto R, Stephenson J, Mohammad S, Siddique I, Verma RR (2015) Risk factors for wound infection in surgery for spinal metastasis. Eur Spine J 24(3):528–532. https://doi.org/10.1007/s00586-013-3127-4
McPhee IB, Williams RP, Swanson CE (1998) Factors influencing wound healing after surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 23(6):726–732. https://doi.org/10.1097/00007632-199803150-00015(discussion 732-723)
Moulding HD, Elder JB, Lis E, Lovelock DM, Zhang Z, Yamada Y, Bilsky MH (2010) Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine 13(1):87–93. https://doi.org/10.3171/2010.3.SPINE09639
Nemelc RM, Stadhouder A, van Royen BJ, Jiya TU (2014) The outcome and survival of palliative surgery in thoraco-lumbar spinal metastases: contemporary retrospective cohort study. Eur Spine J 23(11):2272–2278. https://doi.org/10.1007/s00586-014-3268-0
Pielkenrood B, Pogoda L, Van Der Velden J, Verkooijen H, Verlaan J, Kasperts N (2018) Pre-versus post-operative radiotherapy: complications after combined therapy for spinal metastases. Radiother Oncol 127:S917. https://doi.org/10.1016/S0167-8140(18)32019-X
Street J, Fisher C, Sparkes J, Boyd M, Kwon B, Paquette S, Dvorak M (2007) Single-stage posterolateral vertebrectomy for the management of metastatic disease of the thoracic and lumbar spine: a prospective study of an evolving surgical technique. J Spinal Disord Tech 20(7):509–520. https://doi.org/10.1097/BSD.0b013e3180335bf7
Wang JC, Boland P, Mitra N, Yamada Y, Lis E, Stubblefield M (2004) Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 1(3):287–298. https://doi.org/10.3171/spi.2004.1.3.0287
Young RF, Post EM, King GA (1980) Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg 53(6):741–748. https://doi.org/10.3171/jns.1980.53.6.0741
Akeyson EW, McCutcheon IE (1996) Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg 85(2):211–220. https://doi.org/10.3171/jns.1996.85.2.0211
Carl HM, Ahmed AK, Abu-Bonsrah N, De la Garza RR, Sankey EW, Pennington Z, Bydon A, Witham TF, Wolinsky JP, Gokaslan ZL, Sacks JM, Goodwin CR, Sciubba DM (2018) Risk factors for wound-related reoperations in patients with metastatic spine tumor. J Neurosurg Spine 28(6):663–668. https://doi.org/10.3171/2017.10.SPINE1765
Itshayek E, Yamada J, Bilsky M, Schmidt M, Shaffrey C, Gerszten P, Polly D, Gokaslan Z, Varga PP, Fisher CG (2010) Timing of surgery and radiotherapy in the management of metastatic spine disease: a systematic review. Int J Oncol 36(3):533–544. https://doi.org/10.3892/ijo_00000527
Springfield DS (1993) Surgical wound healing. In: Verweij J, Pinedo HM, Suit HD (eds) Multidisciplinary treatment of soft tissue sarcomas. Springer, Boston, MA, pp 81–98
Wang J, Boerma M, Fu Q, Hauer-Jensen M (2006) Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia 10(6):502–506. https://doi.org/10.1007/s10029-006-0150-y
Shamberger R (1985) Effect of chemotherapy and radiotherapy on wound healing: experimental studies. In: Metzger U, Largiadèr F, Senn HJ (eds) Perioperative chemotherapy. Springer, Berlin, Heidelberg, pp 17–34
Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X (1998) Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol 17(2):117–123
Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG (2012) Wound healing after radiation therapy: review of the literature. Radiat Oncol 7(1):162. https://doi.org/10.1186/1748-717X-7-162
Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT (1999) Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 24(18):1943–1951. https://doi.org/10.1097/00007632-199909150-00014
Paulino Pereira NR, Ogink PT, Groot OQ, Ferrone ML, Hornicek FJ, van Dijk CN, Bramer JAM, Schwab JH (2019) Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J 19(1):144–156. https://doi.org/10.1016/j.spinee.2018.05.037
Laohacharoensombat W, Wongwai T, Wajanavisit W (1997) Spinal metastasis: results of surgical management. J Orthop Surg Hong Kong 5:3–10
Berriochoa CA, Bennett EE, Miller JA, Balagamwala EH, Ward MC, Chao ST, Suh JH, Benzel EC, Soeder S, Yu N, Manyam B, Angelov L (2016) A comparison of oncologic and toxicity outcomes in patients receiving conventional external beam radiation therapy versus SBRT to instrumented spinal fields. Int J Radiat Oncol Biol Phys 96(2):E113–E114. https://doi.org/10.1016/j.ijrobp.2016.06.877
Adams EJ, Warrington AP (2008) A comparison between cobalt and linear accelerator-based treatment plans for conformal and intensity-modulated radiotherapy. Br J Radiol 81(964):304–310. https://doi.org/10.1259/bjr/77023750
Redmond KJ, Sciubba D, Khan M, Gui C, S-fL Lo, Gokaslan ZL, Leaf B, Kleinberg L, Grimm J, Ye X, Lim M (2020) A phase 2 study of post-operative stereotactic body radiation therapy (SBRT) for solid tumor spine metastases. Int J Radiat Oncol Biol Phys 106(2):261–268. https://doi.org/10.1016/j.ijrobp.2019.10.011
Roesch J, Cho JBC, Fahim DK, Gerszten PC, Flickinger JC, Grills IS, Jawad M, Kersh R, Letourneau D, Mantel F, Sahgal A, Shin JH, Winey B, Guckenberger M (2017) Risk for surgical complications after previous stereotactic body radiotherapy of the spine. Radiat Oncol 12(1):153. https://doi.org/10.1186/s13014-017-0887-8
Chen K, Huang L, Cai Z, Shi J, You K, Shen H (2017) Micro-invasive surgery combined with intraoperative radiotherapy for the treatment of spinal metastasis. Eur Spine J 26(7):1893–1901. https://doi.org/10.1007/s00586-016-4826-4
Kumar N, Zaw AS, Reyes MR, Malhotra R, Wu PH, Makandura MC, Thambiah J, Liu GK, Wong HK (2015) Versatility of percutaneous pedicular screw fixation in metastatic spine tumor surgery: a prospective analysis. Ann Surg Oncol 22(5):1604–1611. https://doi.org/10.1245/s10434-014-4178-4
Kumar N, Malhotra R, Maharajan K, Zaw AS, Wu PH, Makandura MC, Po Liu GK, Thambiah J, Wong HK (2017) Metastatic spine tumor surgery: a comparative study of minimally invasive approach using percutaneous pedicle screws fixation versus open approach. Clin Spine Surg 30(8):E1015–e1021. https://doi.org/10.1097/bsd.0000000000000400
O'Toole JE, Eichholz KM, Fessler RG (2009) Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 11(4):471–476. https://doi.org/10.3171/2009.5.Spine08633
Lu VM, Alvi MA, Goyal A, Kerezoudis P, Bydon M (2018) The potential of minimally invasive surgery to treat metastatic spinal disease versus open surgery: a systematic review and meta-analysis. World Neurosurg 112:e859–e868. https://doi.org/10.1016/j.wneu.2018.01.176
Lee RS, Batke J, Weir L, Dea N, Fisher CG (2018) Timing of surgery and radiotherapy in the management of metastatic spine disease: expert opinion. J Spine Surg 4(2):368–373. https://doi.org/10.21037/jss.2018.05.05
Yokogawa N, Murakami H, Demura S, Kato S, Yoshioka K, Hayashi H, Ishii T, Igarashi T, Fang X, Tsuchiya H (2014) Perioperative complications of total en bloc spondylectomy: adverse effects of preoperative irradiation. PLoS ONE 9(6):e98797. https://doi.org/10.1371/journal.pone.0098797
Barzilai O, McLaughlin L, Lis E, Yamada Y, Bilsky MH, Laufer I (2019) Outcome analysis of surgery for symptomatic spinal metastases in long-term cancer survivors. J Neurosurg Spine 31(2):1–6. https://doi.org/10.3171/2019.2.SPINE181306
Abu-Bonsrah N, Goodwin CR, De la Garza-Ramos R, Sankey EW, Liu A, Kosztowski T, Elder BD, Bettegowda C, Bydon A, Witham TF, Wolinsky JP, Gokaslan ZL, Sciubba DM (2017) Readmissions after surgical resection of metastatic tumors of the spine at a single institution. World Neurosurg 101(695–701):e691. https://doi.org/10.1016/j.wneu.2017.02.065
Harel R, Emch T, Chao S, Elson P, Krishnaney A, Djemil T, Suh J, Angelov L (2016) Quantitative evaluation of local control and wound healing following surgery and stereotactic spine radiosurgery for spine tumors. World Neurosurg 87:48–54. https://doi.org/10.1016/j.wneu.2015.10.075
Massicotte E, Foote M, Reddy R, Sahgal A (2012) Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat 11(1):15–25. https://doi.org/10.7785/tcrt.2012.500230
Harel R, Chao S, Krishnaney A, Emch T, Benzel EC, Angelov L (2010) Spine instrumentation failure after spine tumor resection and radiation: comparing conventional radiotherapy with stereotactic radiosurgery outcomes. World Neurosurg 74(4–5):517–522. https://doi.org/10.1016/j.wneu.2010.06.037
Fourney DR, Abi-Said D, Lang FF, McCutcheon IE, Gokaslan ZL (2001) Use of pedicle screw fixation in the management of malignant spinal disease: experience in 100 consecutive procedures. J Neurosurg 94(1 SUPPL.):25–37. https://doi.org/10.3171/spi.2001.94.1.0025
Johnston FG, Uttley D, Marsh HT (1989) Synchronous vertebral decompression and posterior stabilization in the treatment of spinal malignancy. Neurosurgery 25(6):872–876. https://doi.org/10.1097/00006123-198912000-00004
Tsagozis P, Bauer HCF (2019) Outcome of surgical treatment for spinal cord compression in patients with hematological malignancy. Int J Spine Surg 13(2):186–191. https://doi.org/10.14444/6025
Roser S, Maharaj MM, Taylor MA, Kuru R, Hansen MA, Ferch R (2019) Vertebrectomy in metastatic spinal tumours: a 10 year, single-centre review of outcomes and survival. J Clin Neurosci 68:218–223. https://doi.org/10.1016/j.jocn.2019.04.032
Iida K, Matsumoto Y, Setsu N, Harimaya K, Kawaguchi K, Hayashida M, Okada S, Nakashima Y (2018) The neurological outcome of radiotherapy versus surgery in patients with metastatic spinal cord compression presenting with myelopathy. Arch Orthop Trauma Surg 138(1):7–12. https://doi.org/10.1007/s00402-017-2817-5
Park HY, Lee SH, Park SJ, Kim ES, Lee CS, Eoh W (2015) Surgical management with radiation therapy for metastatic spinal tumors located on cervicothoracic junction: a single center study. J Korean Neurosurg Soc 57(1):42–49. https://doi.org/10.3340/jkns.2015.57.1.42
Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, Wolinsky JP, Gokaslan Z (2008) Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery 62(3):683–692. https://doi.org/10.1227/01.neu.0000317317.33365.15(discussion 683-692)
Sundaresan N, Sachdev VP, Holland JF, Moore F, Sung M, Paciucci PA, Wu LT, Kelligher K, Hough L (1995) Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol 13(9):2330–2335. https://doi.org/10.1200/JCO.1995.13.9.2330
Harrington KD (1988) Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop Relat Res 233:177–197
Acknowledgements
We would like to acknowledge Dr Nivetha Ravikumar and Alathur Ramakrishnan Sridharan for their contribution in data collection.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Search strategies used to search PubMed, Scopus and Embase databases
PubMed search strategy
(((((("spine"[MeSH Terms] OR "spine"[All Fields]) OR "Spinal"[All Fields]) OR "Vertebra*"[All Fields]) AND ((((((("neoplasms"[MeSH Terms] OR "neoplasms"[All Fields]) OR ("neoplasm metastasis"[MeSH Terms] OR ("neoplasm"[All Fields] AND "metastasis"[All Fields]) OR "neoplasm metastasis"[All Fields])) OR "Cancer"[All Fields]) OR "Malignan*"[All Fields]) OR "Metastases"[All Fields]) OR "Metastasis"[All Fields]) OR "Metastatic"[All Fields])) AND (((("laminectomy"[MeSH Terms] OR "laminectomy"[All Fields]) OR ("surgery"[Subheading] OR "surgery"[All Fields] OR "surgical procedures, operative"[MeSH Terms] OR ("surgical"[All Fields] AND "procedures"[All Fields] AND "operative"[All Fields]) OR "operative surgical procedures"[All Fields] OR "surgery"[All Fields] OR "general surgery"[MeSH Terms] OR ("general"[All Fields] AND "surgery"[All Fields]) OR "general surgery"[All Fields])) OR "Vertebrectomy"[All Fields]) OR "Corpectomy"[All Fields])) AND (((("radiotherapy"[Subheading] OR "radiotherapy"[All Fields] OR "radiotherapy"[MeSH Terms]) OR "Radiation therapy"[All Fields]) OR "Irradiation"[All Fields]) OR "Stereotactic"[All Fields])) AND (((((((("wounds and injuries"[MeSH Terms] OR ("wounds"[All Fields] AND "injuries"[All Fields]) OR "wounds and injuries"[All Fields]) OR ("wound healing"[MeSH Terms] OR ("wound"[All Fields] AND "healing"[All Fields]) OR "wound healing"[All Fields])) OR ("wound infection"[MeSH Terms] OR ("wound"[All Fields] AND "infection"[All Fields]) OR "wound infection"[All Fields])) OR "Wound problem"[All Fields]) OR "Wound complication"[All Fields]) OR "Wound dehiscence"[All Fields]) OR "Major wound complication"[All Fields]) OR "Wound breakdown"[All Fields]) AND (has abstract[text] AND "humans"[MeSH Terms] AND English[lang] AND ("adolescent"[MeSH Terms] OR "adult"[MeSH Terms]))
Scopus search strategy
(TITLE-ABS-KEY (spine OR "Spinal" OR "Vertebra*")) AND (TITLE-ABS-KEY (neoplasms OR neoplasm AND metastasis OR "Cancer" OR "Malignan*" OR "Metastases" OR "Metastasis" OR "Metastatic")) AND (TITLE-ABS-KEY (laminectomy OR surgery OR "Vertebrectomy" OR "Corpectomy")) AND (TITLE-ABS-KEY (radiotherapy OR "Radiation therapy" OR "Irradiation" OR "Stereotactic")) AND (TITLE-ABS-KEY (wounds AND injuries OR wound AND healing OR wound AND infection OR "Wound problem" OR "Wound complication" OR "Wound dehiscence" OR "Major wound complication" OR "Wound breakdown")) AND (LIMIT-TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018) OR LIMIT-TO (PUBYEAR, 2017) OR LIMIT-TO (PUBYEAR, 2016) OR LIMIT-TO (PUBYEAR, 2015) OR LIMIT-TO (PUBYEAR, 2014) OR LIMIT-TO (PUBYEAR, 2013) OR LIMIT-TO (PUBYEAR, 2012) OR LIMIT-TO (PUBYEAR, 2011) OR LIMIT-TO (PUBYEAR, 2010) OR LIMIT-TO (PUBYEAR, 2009) OR LIMIT-TO (PUBYEAR, 2008) OR LIMIT-TO (PUBYEAR, 2007) OR LIMIT-TO (PUBYEAR, 2006) OR LIMIT-TO (PUBYEAR, 2005) OR LIMIT-TO (PUBYEAR, 2004) OR LIMIT-TO (PUBYEAR, 2003) OR LIMIT-TO (PUBYEAR, 2002) OR LIMIT-TO (PUBYEAR, 2001) OR LIMIT-TO (PUBYEAR, 1999) OR LIMIT-TO (PUBYEAR, 1998) OR LIMIT-TO (PUBYEAR, 1997) OR LIMIT-TO (PUBYEAR, 1996) OR LIMIT-TO (PUBYEAR, 1991) OR LIMIT-TO (PUBYEAR, 1989) OR LIMIT-TO (PUBYEAR, 1986) OR LIMIT-TO (PUBYEAR, 1984) OR LIMIT-TO (PUBYEAR, 1982) OR LIMIT-TO (PUBYEAR, 1971)) AND (LIMIT-TO (LANGUAGE, "English")).
Embase search strategy
('spine'/exp OR 'spine':ab,kw,ti OR spinal:ab,kw,ti OR vertebra*:ab,kw,ti) AND (neoplasms:ab,kw,ti OR (neoplasm AND metastasis:ab,kw,ti) OR cancer:ab,kw,ti OR malignancy:ab,kw,ti OR metastases:ab,kw,ti OR metastasis:ab,kw,ti OR metastatic:ab,kw,ti OR 'neoplasm'/exp OR 'neoplasm metastasis'/exp) AND (laminectomy:ab,kw,ti OR surgery:ab,kw,ti OR vertebrectomy:ab,kw,ti OR corpectomy:ab,kw,ti OR 'laminectomy'/exp OR 'surgery'/exp) AND ('radiotherapy'/exp OR radiotherapy:ab,kw,ti OR (radiation AND therapy:ab,kw,ti) OR irradiation:ab,kw,ti OR stereotactic:ab,kw,ti) AND ('wounds and injuries'/exp OR 'wound healing'/exp OR 'wound infection'/exp OR (wounds AND injuries:ab,kw,ti) OR (wound AND healing:ab,kw,ti) OR (wound AND infection:ab,kw,ti) OR (wound AND problem:ab,kw,ti) OR (wound AND complication:ab,kw,ti) OR (wound AND dehiscence:ab,kw,ti) OR (major AND wound AND complication:ab,kw,ti) OR (wound AND breakdown:ab,kw,ti)) AND ([adult]/lim OR [young adult]/lim) AND ('clinical article'/de OR 'clinical trial'/de OR 'human'/de) AND (1969:py OR 1978:py OR 1983:py OR 1984:py OR 1985:py OR 1986:py OR 1988:py OR 1989:py OR 1990:py OR 1991:py OR 1992:py OR 1993:py OR 1994:py OR 1995:py OR 1996:py OR 1997:py OR 1998:py OR 1999:py OR 2000:py OR 2001:py OR 2002:py OR 2003:py OR 2004:py OR 2005:py OR 2006:py OR 2007:py OR 2008:py OR 2009:py OR 2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py
Appendix 2: Description of the articles from the systematic literature review considered for data extraction
First author, year | Study design | No. of patients (included in final analysis); Gender (N) (M/F) | Age (years) | Treatment sequence (RT to MSTS or MSTS to RT) and type of surgery (N) | Interval between RT and MSTS or vice versa | Results related to wound complications | Conclusion/s |
|---|---|---|---|---|---|---|---|
Redmond KJ, 2020 [53] | Phase II, prospective study | 35 (M/F: 22/13) | Median: 63 (21–75) | Surgical resection prior to SBRT treatment | Surgical resection not more than 16 weeks prior to SBRT | No incidence of wound dehiscence/complications | Post-operative SBRT demonstrates excellent local control with low toxicity compared to historical post-operative conventional RT. No information on association between post-operative SBRT and wound complications |
Barzilai O, 2019 [62] | Retrospective chart and imaging review | 88 (M/F: 44/44) | Mean: 61 (27–84) | Open posterolateral decompression and stabilisation (67): Post-operative RT (63) (SBRT [63%]; conventional EBRT [33%]); surgery on previously irradiated lesions (20) Percutaneous minimally invasive surgery (21): Post-operative RT (11) | Not mentioned | 3 wound infections or dehiscence requiring additional surgical intervention at 17, 22, and 29 months after initial surgery | Hybrid therapy with separation surgery and post-operative SBRT provides durable local control. Delayed complications include local and marginal tumour recurrence/progression, hardware failure, and wound complications. No information was reported on the correlation between pre- and/or post-operative RT and wound complications. |
Tsagozis P, 2019 [69] | Retrospective review of prospective database | 50 (M/F: 31/19) | Median: 65 (31–85) | Instrumentation (29) Posterior decompression only (21) Overall pre- or post-operative RT (33) | Post-operative RT was given 3–6 weeks after wound healing. | 5 wound infections and dehiscence; 2 required extraction of the implant combined with antibiotic therapy; 2 needed debridement and flap reconstruction. | Early surgical decompression is associated with good neurological outcome in patients with cord compression from radiosensitive haematological neoplasia. A significant association was reported between RT and post-operative complications, with no mention of any specific connection of RT to wound complications. |
Szczerba P, 2019 [24] | Retrospective analysis | 723 (M/F: 260/463) | Mean age of women: 64 (28–85) Mean age of men: 69 (34–83) | Minimally invasive procedure (vertebroplasty, kyphoplasty, percutaneous stabilisation) (138) Posterior approach (417); anterior approach (137); combined approach (31) Prior RT (n = 151; 21%) | Not mentioned | 42 wound healing problems and infections. Wound-healing disorders and infections were noted more frequently in patients with prior RT (n = 30; 19% of previous RT group). | Previous systemic (radiation) therapy may have a significant impact on the risk of wound-healing complications. No information on the influence of RT pre- or post-surgery on the risk of wound complications |
Roser S, 2019 [70] | Retrospective review | 137 (M/F: 80/57) (141 vertebrectomies) | Mean: 61 (25–83) | Vertebrectomy with anterior reconstruction and posterior fixation Thoracic (104); Lumbar (2); and Cervical (14) Pre-operative RT details were collected, but not reported. | Not mentioned | 7 wound infections or dehiscence requiring operative revision. Out of these, 4 underwent pre-operative RT. | Vertebrectomy is a safe and effective means of providing circumferential neural decompression and stabilisation with an acceptable complication rate in patients with vertebral metastases. No information has been reported on the association between RT and wound complications. |
Paulino Pereira NR, 2019 [49] | Retrospective cohort study | 647 (M/F: 375/272) | Median: 60 (52–68) | Corpectomy with stabilisation (313); decompression with stabilisation (230); decompression alone (84); stabilisation alone (20) Prior RT (221) | Not mentioned | Wound infections and dehiscence were noted in 42 and 28 patients, respectively resulting in reoperation. Prior RT was significantly associated with reoperation. | Prior RT to the spinal tumour was independently associated with reoperation. Information regarding association between RT and wound complications has not been reported. |
Pielkenrood B, 2018 [36] | Prospective cohort study | 142 (M/F: not mentioned) | Not mentioned | Surgical stabilisation Pre-operative RT (91) Post-operative RT (51) | Interval between: Pre-operative RT and surgery: 0–280 days; surgery and post-operative RT: 8–69 days | 10 wound complications; 3 in pre-operative RT group, and 7 in post-operative RT group | There was no significant difference in the complication rates, including wound complications between pre- and post-operative RT groups for spinal metastases. |
Carl HM, 2018 [41] | Retrospective review | 159 (M/F: 85/74) | Average: 59.6 ± 11.7 | Resection of metastatic spine lesions: Anterior approach (41); Posterior approach (105); Combined approach (13) Pre-operative RT (63) Post-operative RT (75): Conventional EBRT (70); SRS (5) | Not mentioned | 22 wound complications (6 wound dehiscence and 16 wound infections requiring surgery): 12 had pre-operative RT and 6 had post-operative conventional EBRT, but no significant association was detected. | Patients who undergo metastatic spine tumour resection are at an inherently high risk for wound-related reoperations due to high rates of adjuvant radiation. No information on association between RT and/or interval between surgery and RT and wound complications was reported. |
Iida K, 2018 [71] | Retrospective analysis | 34 (M/F: 17/17) | Mean: 64 (45–87) | Laminectomy of the compressed spinal cord and posterior instrument stabilisation; with or without RT All patients who underwent surgery + RT had post-operative RT | Not mentioned | 3 wound complications | Surgical decompression and stabilisation may be required to improve the neurological function in patients with metastatic spinal cord compression presenting with myelopathy. High rate of complications associated with surgery should be considered. No information was reported on the association between RT and wound complications. |
Versteeg AL, 2018 [23] | Prospective cohort | 13 (M/F: Not mentioned) | Range: 41–85 | SBRT followed by surgical stabilisation within 24 h Surgery: Percutaneous pedicle screw fixation (11); open procedure including decompression (2) | Median time between SBRT and surgery was 17 h (IQR 5–19 h) with four patients receiving both treatments on the same day. | None of the patients experienced disturbed wound healing or wound infection. | This study demonstrates the safety and feasibility of SBRT followed by surgery within 24 h for the treatment of spinal metastases with no patients demonstrating wound complications. |
Abu-Bonsrah N, 2017 [63] | Retrospective analysis | 159 (M/F: 85/74) | Mean: 59.6 ± 11.7 | Surgical resection of metastatic spine tumour: Anterior approach (41); Posterior approach (105); Combined approach (13) Prior RT (62) | Not mentioned | 76 (47.8%) had at least one peri-operative complication, of which 10 (6.3%) had wound complications. | Prior RT was independently associated with an increased risk for readmissions, but no information on association between prior RT and readmissions due to wound complications. |
Roesch J, 2017 [54] | Retrospective study | 30 (M/F: 13/17) | Median: 59 (27–84) | Open surgical decompression (24) and/or pedicle screw stabilisation (18) Vertebroplasty or balloon kyphoplasty only (10) (5 patients had a combined approach with decompression and placement of spinal instrumentation) Previous SBRT in all patients (conventional RT in 17 patients prior to SBRT) | Median interval between SBRT and surgical salvage therapy: 6 months (1–39 months) | 2 cases of delayed wound healing | Prior spine SBRT does not significantly increase the risk of intra- and post-surgical complications. No information on the association between prior RT and wound complications has been reported. |
Chen K, 2017 [55] | Retrospective cohort study | 40 (M/F: 18/22) | Mean: 47.3 (28–67) | 32 patients (42 vertebrae) had percutaneous puncture IORT + Cement; 8 patients (10 vertebrae) had open decompression + IORT All patients received IORT and 8 patients received post-operative EBRT. | Not mentioned | No wound complications | Surgery combined with tumour conformal IORT for thoracolumbar metastases has good short-term effects with no apparent wound complications. |
Berriochoa CA, 2016 [51] | Retrospective review | 63 (M/F: Not mentioned) | Not mentioned | Surgical decompression (corpectomy or separation surgery) followed by palliative RT Post-operative SBRT (37); post-operative EBRT (26) Instrumentation was performed in all 37 SBRT, and 18/26 EBRT patients. | RT was performed within 3 months of surgery. | 2 wound complications in SBRT group and 1 in EBRT group. One-year projected cumulative incidence of wound complications in the SBRT and EBRT groups: 2.8% and 6.3%, respectively | Both post-operative SBRT and EBRT are safe for spinal metastases. No information on association between post-operative RT and wound complications was reported. |
Harel R, 2016 [64] | Retrospective cohort study | Total N = 22; Response to therapy was evaluated in 17 patients (M/F: 11/6). | Mean: 57 (7–85) | Conventional approach and post-operative single-session SRS (22) Combined ventral and dorsal approach (5); ventral (13); dorsal (4) Decompression only (6); un-instrumented fusion (1); Decompression + instrumented fusion (15) | SRS done within 2 months following surgery | 2 superficial wound infections; pre-SRS, no prior RT. No cases of new/persistent wound infection or dehiscence post SRS | Spine surgery with adjuvant SRS appears to be beneficial in reducing potential wound complications. |
Sugita S, 2016 [26] | Retrospective cohort study | 279 (M/F: 118/161) | Median: 63 (56–70) | All patients received IORT and surgery (post fusion surgery [with or without decompression]); in addition, 137 received pre-operative RT. | Not mentioned | 27 of 137 who received pre-op RT had wound infection. | Pre-operative RT was found to be independently associated with occurrence of SSI in patients who underwent surgery with IORT for spinal metastases. |
Park HY, 2015 [72] | Retrospective chart review | 23 (M/F: 17/6) | Mean: 54.72 (35–73) | Simple decompression with laminectomy for tumour involved posterior column, laminectomy plus trans-pedicular anterior decompression for tumour located vertebral body and total spondylectomy. For anterior column support, corpectomy followed by inserting titanium mesh cage or cement bloc. All procedures were combined with instrumentation Pre-operative RT (6); Post-operative RT (10); Pre- and post-operative RT (3) | Not mentioned | 3 wound infections or dehiscence (one had pre- and one had post-operative RT); two required re-operation | Surgical procedures including posterior approach, debulking surgery, and stabilisation under neurophysiologic monitoring with adjuvant radiation therapy are an important part of the treatment of metastatic spinal tumours located on cervicothoracic junction. No information on the association between pre- or post-operative RT and wound complications has been reported. |
Yokogawa N, 2014 [61] | Retrospective cohort study | 50 (M/F: 27/23) | Mean: 57.8 (24–75) | Surgical approach: TES Pre-operative RT (18) | < 12 months in 8 patients, > 12 months in 10 patients | No wound complications | Perioperative complication rate associated with TES for spinal metastasis is significantly higher in pre-operative RT group than in no RT group. |
Keam J, 2014 [31] | Retrospective cohort study | 165 (165 surgical wounds) (M/F: 90/75) | Median: XRT: 62 (26–84); IGRT: 60 (43–82) | Single-stage surgery with decompression and spinal fixation Pre-operative XRT (130) Pre-op hypo fractionated IGRT (35) | Mean: XRT: 410 ± 658 days; IGRT: 345 ± 389 days | 24 wound complications: 22 were after XRT, and 2 were after hypo fractionated IGRT | No significant difference in wound complication rates in patients receiving conventionally fractionated or high dose hypo fractionated radiation before surgery. |
Nemelc RM, 2014 [35] | Retrospective cohort study | Total, N = 86 (Survival analysis done on 81 patients) (M/F: 37/44) | 59 (24–87) | Anterior approach (11); posterior approach (67); Combined approach (8) Details of RT: Not mentioned | Not mentioned | 4 cases of deep surgical wound infection, out of which 2 received RT prior to surgery and 1 patient received adjuvant RT | Pre-operative RT had no influence on the risk for post-operative surgical wound infections. |
Kumar S, 2015 [32] | Retrospective cohort study | 95 (M/F: 51/44) | 19–87 | Combined anterior–posterior surgical approach; reconstruction (6) No patients underwent RT in the immediate pre or post-operative period. | Post-operative RT was commenced once the surgical wound was completely healed. | All the patients with wound infection had it in their post-spinal wounds. No wound infection was noted after the onset of post-operative RT. | ≥ 7 vertebral levels of surgery increase the risk of infection significantly. No correlation was observed between RT and wound infection. |
Massicotte E, 2012 [65] | Retrospective cohort study | 10 (M/F: Not mentioned) | Not Mentioned | All patients treated with MASS + adjuvant post-operative SBRT Pre-operative RT (1) | Median time to SBRT planning: 6.5 days (1–18) and treatment ensued on average 7 days following planning | No wound complications | MASS avoids the risks of surgical morbidity and the potential delays in receiving post-operative RT/chemo associated with invasive surgery. |
Omeis IA, 2011 [28] | Retrospective, case–control | 678 patients (M/F: 364/314) (895 procedures) | Mean: 47.2 | Treatment sequence not mentioned Surgery: 82% patients were treated by posterior approach. | Not mentioned | 65 patients developed SSI. | Having a previous surgery or RT to the operative area was a significant risk for wound infection. No significant difference was noted in wound infections based on surgical approach (anterior vs. posterior). |
Moulding HD, 2010 [34] | Retrospective cohort study | 21 (M/F: 15/6) | Median: 53.2 (28.7–78.4); mean: 52.9 ± 12.1 | Posterolateral decompression and instrumentation with local autologous bone graft in all patients Adjuvant post-operative single-fraction high-dose SRS | Mean duration from surgery to SRS: 43.9 (26–63) days | No wound complications | High-dose single-fraction radiation (24 Gy) provides durable tumour control that is histology independent. No correlation was observed between RT and wound infection. |
Harel R, 2010 [66] | Retrospective cohort study | 15 (M/F: 10/5) | Mean: 53 (24–79) | Surgery + post-operative RT with XRT or SRS Combined anterior and posterior approach (4); single approach (anterior or posterior) (11) | Surgery to post-operative RT: Within 2 months (median time from surgery to SRS was 38 days) | One patient in the XRT group had a deep wound infection. | SRS is a feasible adjuvant treatment to spine surgery with high rates of tumour control and low associated complications. |
Demura Sa, 2009 [29] | Retrospective cohort study/prospective cohort study | 110 (M/F: 65/45) (113 surgeries) | Median: 56 (32–72) | En bloc surgery (38); Debulking (43); Palliative (29) Pre-operative RT (22) | (Mean ± SD): Without PGE1: 12.1 ± 9.1 months; With PGE1: 15.1 ± 14.5 months | SSI incidence: 7.1% (8/113 surgeries); rate of SSI with irradiation: 31.8% (7/22 cases) and without irradiation (1.1%, 1/91 cases) | Prior irradiation was associated with a higher incidence of SSI by univariate analysis and multivariate logistic regression. |
Chaichana KL, 2008 [73] | Retrospective review | 78 (M/F: 46/32) | Mean: 56 ± 13 | Anterior approach (28); Posterior approach (43); Anteroposterior approach (7) Post-operative RT (21); no details on pre-operative RT | Not mentioned | 4 wound dehiscence | Pre- and post-operative RT were associated with a lower and higher likelihood of regaining ambulatory function at follow-up, respectively. No information on association between RT and wound complications was reported. |
Street J, 2007 [37] | Prospective cohort study | 66 (SPLV group of 42) (M/F: 23/19) | Mean: 56.04 (19–76) | SPLV group (42): Simultaneous 360° tumour resection and immediate spinal column reconstruction. 14 patients had pre-operative RT/ SPLV for disease of the lumbar spine, combined with bilateral costotransversectomies in the thoracic spine. Comparison group (24): thoracic or lumbar tumour decompression and spinal stabilisation | RT to surgery interval > 7 days | 10 patients with major wound complications, of which 3 had had pre-operative RT | Pre-operative RT did not adversely affect the rate of complications in either population of patients. |
Holman PJ, 2005 [30] | Retrospective cohort study | 139 (M/F: 85/54) (166 procedures) | Median: 55 (12–83) | 89 patients received some form of treatment for spinal disease at outside institutions. RT (14); surgical decompression (4); RT + surgery (8); RT + Chemo + Surgery (9). 80 patients required post-operative adjuvant therapy including RT alone and RT and chemo Surgical approach: Thoracoabdominal (27); retroperitoneal (47); transperitoneal (1); transpedicular (23) | Not mentioned | 7 wound infections, majority of which (6/7) were in posterior surgeries | No significant association between pre-operative RT and wound infection. Whenever surgical intervention is contemplated, RT involving the operative field should be delayed, if possible. |
Wang JC, 2004 [38] | Retrospective cohort study | 140 (M/F: 105/35) | Median: 60.3(20.5–85.9) | Single-stage posterolateral transpedicular approach Pre-operative RT (84); patients with radioresistant tumours (14) had surgery within 30 days of pre-operative RT for tumour progression Post-operative EBRT (24): Operation + failed EBRT—underwent repeated IMRT (2); no pre-operative RT but had post-operative IMRT (5) | Mean time between failed RT and surgery: 4.2 months | Most prevalent complication: Wound dehiscence and/or infection—16 patients (11.4%) | No statistical difference was noted in risk rates in patients undergoing pre-operative RT or de novo surgery (p = 0.21), including patients who had pre-operative RT within 6 weeks of surgery (p = 0.29). |
Sundaresan N, 2002 [25] | Retrospective cohort study | 80 (M/F: 44/36) | Mean: 56; median: 55(22–81) | Surgical approach: anterior (32); strictly posterior or posterolateral (8); anteroposterior (40); en bloc resection (6) Previous RT (40); all had failed RT Remaining 40 patients—surgery because of clinical symptoms and the solitary nature of the tumour | Not mentioned | 1/40 de-novo surgery had wound infection. 10/40 previous RT had wound breakdowns. All wound complications requiring revision surgery were noted in irradiated patients. | Wound infection was observed mainly in patients who received pre-operative RT. Statistically significant (p < 0.03) difference was noted in complication rates between irradiated and non-irradiated patients. |
Ghogawala Z, 2001 [22] | Retrospective case series | 85 (M/F: Not mentioned) | Mean: XRT = 55 XRT/ Surgery = 63 Surgery/XRT = 62 | Single-stage posterolateral decompression and stabilisation RT Only (23); pre-operative RT (28); post-operative RT (34) | Pre-operative RT ≤ 7 days in 13 patients; Pre-operative RT > 7 days in 15 patients | 13 major wound complications; pre-operative RT ≤ 7 days: 46% wound complications; pre-operative RT > 7 days: 20% wound complications | Wound complication rate was higher when surgery was performed ≤ 7 days of RT, as compared to > 7 days after RT. |
Fourney DR, 2001 [67] | Retrospective cohort study | 95 patients (M/F: 55/40) (100 procedures) | Median: 54 (14–79) | Surgical approach: post tumour resection and stabilisation (48); anterior approach + posterior reconstruction (51); others (1) Pre-operative RT only (9); chemo only (34); RT and chemo (34); Neither (18) Post-operative RT only (7); chemo only (23); RT and chemo (20); Neither (35) | Not mentioned | 9 wound infections | Pre-operative RT (p = 0.002) was associated with post-operative complications. The study findings emphasize the importance of avoiding pre-operative RT in patients who are candidates for surgery as a first-line therapy. |
Wise JJ, 1999 [48] | Retrospective cohort study | 80 (M/F: 38/42) (88 procedures) | Median: 55.6 (20–84) | Surgical approach: Anterior, posterior or combined decompression and stabilisation Pre-operative RT (41) (45 procedures); no pre-operative RT (39) (43 procedures) | Not mentioned | Superficial wound infection (2); deep wound infection (6); all these patients had undergone pre-operative RT | Wound infection was observed mainly in patients who received pre-operative RT. |
Pascal-Mousellard H, 1998 [27] | Retrospective cohort study | 145 | Not Mentioned | Treatment sequence not mentioned Surgical approach: Cervical corpectomy (48); thoracic laminectomy (109) | Not mentioned | Post-operative wound infection (6): 4 had pre-operative RT; wound dehiscence (8): 7 had pre-operative RT; delayed healing (6): 4 had pre-operative RT | Wound infection rate in pre-operative RT patients was higher than those with post-operative RT (statistically significant). Vertebral metastases should be primarily treated by surgery before RT in order to significantly prevent surgical complications. |
McPhee B, 1998 [33] | Retrospective review | 53 (75 wounds) (M/F: 31/22) | Mean: patients with wound infection/dehiscence: 54 ± 13; patients with healed wounds: 53 ± 17 | Anterior surgery (23); posterior surgery (52) Perioperative RT was administered in 10 wounds that broke down and 32 wounds that healed | 1 month before or 1 month after surgery | Of the 75 wounds, 60 healed and 15 broke as a result of infection or dehiscence. | There was no statistically significant correlation between perioperative RT and wound infections. |
Akeyson EW, 1996 [40] | Retrospective case series | 25 (M/F: 13/12) | Mean: 53.8 ± 2.6 | Single-stage operation combining a near complete (90%) single- or multi-level spondylectomy, vertebral body replacement using methyl methacrylate (MMA), and posterior fixation Pre-operative RT (20); post-operative RT (3) after allowing enough time for wound healing | Not mentioned | 3 non-healing wounds (1 infection and dehiscence; 1 dehiscence without infection; 1 infection). 2 out of these 3 patients received pre-operative RT leading to wound-healing problems. No deep wound infections | Wound infection was observed mainly in patients who received pre-operative RT. |
Sundaresan N, 1995 [74] | Retrospective study | 110 (M/F: 57/53) | Median: 58 (28–85) | Staged anterior–posterior resection and instrumentation (53); anterior resection with instrumentation (33); anterior resection only (18); posterior resection and instrumentation (6) Previous RT (47); post-operative RT (20) | Not mentioned | 18 wound breakdown and 13 infections | Anterior–posterior resection of neoplastic compression from epidural metastasis is effective, with majority requiring instrumentation. No information on association between prior or post RT and wound complications was reported. |
Johnston FG, 1989 [68] | Retrospective cohort study | 34 (M/F: 17/17) | Mean: 61 (18–78) | Single-stage anterior decompression and debulking, with posterior stabilisation Pre-operative RT failed to maintain neurological status (14) | Not mentioned | Wound infection and dehiscence in 5/34 (15%) patients; all 5 had pre-operative RT | Wound infection was observed mainly in patients who received pre-operative RT. |
Harrington KD, 1988 [75] | Retrospective cohort study | 77 (92 operations) | Not mentioned | Anterior decompression alone (76); anterior decompression + second-stage posterior decompression and stabilisation (3–10 days after first surgery) (5) Pre-operative RT (58); post-operative RT (4) | Post-op RT 3 weeks after surgery (in 4 patients who were not previously irradiated) | 1 deep infection in 83 anterior stabilisation procedures. 3 wound dehiscence in 6 patients with posterior stabilisation after pre-operative RT. All 3 had secondary wound infection. | Wound infection was observed mainly in patients who received pre-operative RT. |
Young RF, 1980 [39] | Randomised controlled trial | 29 (M/F: not mentioned) | Mean: Group I: 53.8 (19–70) Group II: 63.8 (34–83) | Decompressive laminectomy + post-operative RT (group I) (16); RT alone (group II) (13) | Group I: post-operative RT: 7 days after surgery | No wound infection/dehiscence following surgery | No statistical difference between surgery + RT vs RT alone in treatment of spinal epidural metastasis. No wound complications were noted following surgery or related to RT |
Rights and permissions
About this article
Cite this article
Kumar, N., Madhu, S., Bohra, H. et al. Is there an optimal timing between radiotherapy and surgery to reduce wound complications in metastatic spine disease? A systematic review. Eur Spine J 29, 3080–3115 (2020). https://doi.org/10.1007/s00586-020-06478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06478-5