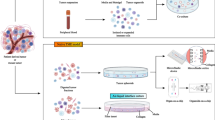
Abstract
Patients with triple negative breast cancer (TNBC) typically receive chemotherapy, surgery, and radiation therapy. Although this treatment improves prognosis for most patients, some patients continue to experience recurrence within 5 years. Preclinical studies have shown that immune cell infiltration at the irradiated site may play a significant role in tumor cell recruitment; however, little is known about the mechanisms that govern this process. This lack of knowledge highlights the need to evaluate radiation-induced cell infiltration with models that have controllable variables and maintain biological integrity. Mammary organoids are multicellular three-dimensional (3D) in vitro models, and they have been used to examine many aspects of mammary development and tumorigenesis. Organoids are also emerging as a powerful tool to investigate normal tissue radiation damage. In this review, we evaluate recent advances in mammary organoid technology, consider the advantages of using organoids to study radiation response, and discuss future directions for the applications of this technique.




Similar content being viewed by others
References
Acerbi, I., L. Cassereau, I. Dean, Q. Shi, A. Au, C. Park, Y. Y. Chen, J. Liphardt, E. S. Hwang, and V. M. Weaver. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7:1120–1134, 2015.
Adkins, F. C., A. M. Gonzalez-Angulo, X. Lei, L. F. Hernandez-Aya, E. A. Mittendorf, J. K. Litton, J. Wagner, K. K. Hunt, W. A. Woodward, and F. Meric-Bernstam. Triple-negative breast cancer is not a contraindication for breast conservation. Ann. Surg. Oncol. 18:3164–3173, 2011.
Adra, J., D. Lundstedt, F. Killander, E. Holmberg, M. Haghanegi, E. Kjellén, P. Karlsson, and S. Alkner. Distribution of locoregional breast cancer recurrence in relation to postoperative radiation fields and biological subtypes. Int. J. Radiat. Oncol. 105:285–295, 2019.
Afghahi, A., N. Purington, S. S. Han, M. Desai, E. Pierson, M. B. Mathur, T. Seto, C. A. Thompson, J. Rigdon, M. L. Telli, S. S. Badve, C. N. Curtis, R. B. West, K. Horst, S. L. Gomez, J. M. Ford, G. W. Sledge, and A. W. Kurian. Higher absolute lymphocyte counts predict lower mortality from early-stage triple-negative breast cancer. Clin. Cancer Res. 24:2851–2858, 2018.
Ahn, G. O., and J. M. Brown. Matrix metalloproteinase-9 Is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 13:193–205, 2008.
Alcaraz, J., R. Xu, H. Mori, C. M. Nelson, R. Mroue, V. A. Spencer, D. Brownfield, D. C. Radisky, C. Bustamante, and M. J. Bissell. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27:2829–2838, 2008.
Al-Mahmood, S., J. Sapiezynski, O. B. Garbuzenko, and T. Minko. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv. Transl. Res. 8:1483–1507, 2018.
Ambroggi, M., E. M. Stroppa, P. Mordenti, C. Biasini, A. Zangrandi, E. Michieletti, E. Belloni, and L. Cavanna. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int. J. Breast Cancer 1–8:2012, 2012.
Andarawewa, K. L., A. C. Erickson, W. S. Chou, S. V. Costes, P. Gascard, J. D. Mott, M. J. Bissell, and M. H. Barcellos-Hoff. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor β-induced epithelial to mesenchymal transition. Cancer Res. 67:8662–8670, 2007.
Avivar-Valderas, A., R. McEwen, A. Taheri-Ghahfarokhi, L. S. Carnevalli, E. L. Hardaker, M. Maresca, K. Hudson, E. A. Harrington, and F. Cruzalegui. Functional significance of co-occurring mutations in PIK3CA and MAP3K1 in breast cancer. Oncotarget 9:21444–21458, 2018.
Ayuso, J. M., A. Gillette, K. Lugo-Cintrón, S. Acevedo-Acevedo, I. Gomez, M. Morgan, T. Heaster, K. B. Wisinski, S. P. Palecek, M. C. Skala, and D. J. Beebe. Organotypic microfluidic breast cancer model reveals starvation-induced spatial-temporal metabolic adaptations. EBioMedicine 37:144–157, 2018.
Bagley, J. A., D. Reumann, S. Bian, J. Lévi-Strauss, and J. A. Knoblich. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14:743–751, 2017.
Bakal, C., G. Church, and N. Perrimon. Regulating Cell Morphology. Science 316:1753–1756, 2007.
Baker, D. G., and R. J. Krochak. The response of the microvascular system to radiation: a review. Cancer Invest. 7:287–294, 1989.
Barcellos-Hoff, M., R. Derynck, M.-S. Tsang, and J. Weatherbee. Transforming growth factor-b activation in irradiated murine mammary gland. J. Clinc. Invest. 92:2065–2072, 1993.
Barcellos-Hoff, M. H., and S. A. Ravani. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60:1254–1260, 2000.
Barreto-Andrade, J. C., E. V. Efimova, H. J. Mauceri, M. A. Beckett, H. G. Sutton, T. E. Darga, E. E. Vokes, M. C. Posner, S. J. Kron, and R. R. Weichselbaum. response of human prostate cancer cells and tumors to combining parp inhibition with ionizing radiation. Mol. Cancer Ther. 10:1185–1193, 2011.
Bertozzi, S., A. P. Londero, C. Cedolini, A. Uzzau, L. Seriau, S. Bernardi, S. Bacchetti, E. M. Pasqual, and A. Risaliti. Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. Springerplus 4:1–8, 2015.
Bierie, B., and H. L. Moses. Tumour microenvironment—TGFΒ: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6:506–520, 2006.
Bochet, L., A. Meulle, S. Imbert, B. Salles, P. Valet, and C. Muller. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem. Biophys. Res. Commun. 411:102–106, 2011.
Borten, M. A., S. S. Bajikar, N. Sasaki, H. Clevers, and K. A. Janes. Automated brightfield morphometry of 3D organoid populations by OrganoSeg. Sci. Rep. 8:1–10, 2018.
Bourhis, J., W. J. Sozzi, P. G. Jorge, O. Gaide, C. Bailat, F. Duclos, D. Patin, M. Ozsahin, F. Bochud, J. F. Germond, R. Moeckli, and M. C. Vozenin. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 139:18–22, 2019.
Boutin, M. E., T. C. Voss, S. A. Titus, K. Cruz-Gutierrez, S. Michael, and M. Ferrer. A high-throughput imaging and nuclear segmentation analysis protocol for cleared 3D culture models. Sci. Rep. 8:1–14, 2018.
Brown, J. M., and J. C. Probert. Long-term recovery of connective tissue after irradiation. Radiology 108:205–207, 1973.
Brown, P. D., S. Pugh, N. N. Laack, J. S. Wefel, D. Khuntia, C. Meyers, A. Choucair, S. Fox, J. H. Suh, D. Roberge, V. Kavadi, S. M. Bentzen, M. P. Mehta, and D. Watkins-bruner. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro. Oncol. 15:1429–1437, 2013.
Camphausen, K., M. A. Moses, C. Ménard, M. Sproull, W. D. Beecken, J. Folkman, and M. S. O’Reilly. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 63:1990–1993, 2003.
Casey, J., X. Yue, T. D. Nguyen, A. Acun, V. R. Zellmer, S. Zhang, and P. Zorlutuna. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 12:1–12, 2017.
Centenera, M. M., T. E. Hickey, S. Jindal, N. K. Ryan, P. Ravindranathan, H. Mohammed, J. L. Robinson, M. J. Schiewer, S. Ma, P. Kapur, P. D. Sutherland, C. E. Hoffman, C. G. Roehrborn, L. G. Gomella, J. S. Carroll, S. N. Birrell, K. E. Knudsen, R. V. Ganesh, L. M. Butler, and W. D. Tilley. A patient-derived explant (PDE) model of hormone-dependent cancer. Mol. Oncol. 12:1608–1622, 2018.
Chatterjee, S., P. Basak, E. Buchel, L. C. Murphy, and A. Raouf. A robust cell culture system for large scale feeder cell-free expansion of human breast epithelial progenitors. Stem Cell Res. Ther. 9:1–10, 2018.
Chen, C. S., M. Mrksich, S. Huang, G. M. Whitesides, and D. E. Ingber. Geometric control of cell life and death. Science 276:1425–1428, 1997.
Chida, S., K. Okada, N. Suzuki, C. Komori, and Y. Shimada. Infiltration by macrophages and lymphocytes in transplantable mouse sarcoma after irradiation with high-intensity focused ultrasound. Anticancer Res. 29:3877–3882, 2009.
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154:274–284, 2013.
Cline, J., G. Dugan, J. Bourland, D. Perry, J. Stitzel, A. Weaver, C. Jiang, A. Tovmasyan, K. Owzar, I. Spasojevic, I. Batinic-Haberle, and Z. Vujaskovic. Post-irradiation treatment with a superoxide dismutase mimic, MnTnHex-2-PyP5+, mitigates radiation injury in the lungs of non-human primates after whole-thorax exposure to ionizing radiation. Antioxidants 7:1–17, 2018.
De Ruysscher, D., G. Niedermann, N. G. Burnet, S. Siva, A. W. M. Lee, and F. Hegi-Johnson. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 13:1–20, 2019.
Dekkers, J. F., M. Alieva, L. M. Wellens, H. C. R. Ariese, P. R. Jamieson, A. M. Vonk, G. D. Amatngalim, H. Hu, K. C. Oost, H. J. G. Snippert, J. M. Beekman, E. J. Wehrens, J. E. Visvader, H. Clevers, and A. C. Rios. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14:1756–1771, 2019.
Demaria, S., B. Ng, M. L. Devitt, J. S. Babb, N. Kawashima, L. Liebes, and S. C. Formenti. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58:862–870, 2004.
Dijkstra, K. K., C. M. Cattaneo, F. Weeber, M. Chalabi, J. van de Haar, L. F. Fanchi, M. Slagter, D. L. van der Velden, S. Kaing, S. Kelderman, N. van Rooij, M. E. van Leerdam, A. Depla, E. F. Smit, K. J. Hartemink, R. de Groot, M. C. Wolkers, N. Sachs, P. Snaebjornsson, K. Monkhorst, J. Haanen, H. Clevers, T. N. Schumacher, and E. E. Voest. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174:1586–1598, 2018.
Dragun, A. E., J. Pan, S. N. Rai, B. Kruse, and D. Jain. Locoregional recurrence in patients with triple-negative breast cancer: preliminary results of a single institution study. Am. J. Clin. Oncol. Cancer Clin. Trials 34:231–237, 2011.
Durante, M., and S. C. Formenti. Radiation-induced chromosomal aberrations and immunotherapy: micronuclei, cytosolic DNA, and interferon-production pathway. Front. Oncol. 8:1–9, 2018.
Ewald, A. J., A. Brenot, M. Duong, B. S. Chan, and Z. Werb. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14:570–581, 2008.
Fajardo, L. F. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 44:13–22, 2005.
Fessenden, T. B., Y. Beckham, M. Perez-Neut, A. H. Chourasia, K. F. Macleod, P. W. Oakes, and M. L. Gardel. Dia1-dependent adhesions are required for invasion by epithelial tissues. J. Cell Biol. 217:1485–1502, 2018.
Feys, L., B. Descamps, C. Vanhove, A. Vral, L. Veldeman, S. Vermeulen, C. De Wagter, M. Bracke, and O. De Wever. Radiation-induced lung damage promotes breast cancer lung-metastasis through CXCR4 signaling. Oncotarget 6:26615–26632, 2015.
Folkman, J., and K. Camphausen. What does radiotherapy do to endothelial cells? Science 293:227–228, 2001.
Furuta, S., G. Ren, J. H. Mao, and M. J. Bissell. Laminin signals initiate the reciprocal loop that informs breast-specific gene expression and homeostasis by activating NO, p53 and microRNAs. Elife 7:1–40, 2018.
Garcia-Barros, M., F. Paris, C. Cordon-Cardo, D. Lyden, S. Rafii, A. Haimovitz-Friedman, Z. Fuks, and R. Kolesnick. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300:1155–1159, 2003.
Grither, W. R., and G. D. Longmore. Inhibition of tumor–microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc. Natl. Acad. Sci. 115:E7786–E7794, 2018.
Groselj, B., J.-L. Ruan, H. Scott, J. Gorrill, J. Nicholson, J. Kelly, S. Anbalagan, J. Thompson, M. R. L. Stratford, S. J. Jevons, E. M. Hammond, C. L. Scudamore, M. Kerr, and A. E. Kiltie. Radiosensitization In vivo by histone deacetylase inhibition with no increase in early normal tissue radiation toxicity. Mol. Cancer Ther. 17:381–392, 2017.
Grudzenski, S., A. Raths, S. Conrad, C. E. Rübe, and M. Löbrich. Inducible response required for repair of low-dose radiation damage in human fibroblasts. Proc. Natl. Acad. Sci. 107:14205–14210, 2010.
Guiet, R., E. Van Goethem, C. Cougoule, S. Balor, A. Valette, T. Al Saati, C. A. Lowell, V. Le Cabec, and I. Maridonneau-Parini. The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. J. Immunol. 187:3806–3814, 2011.
Hacker, B. C., J. D. Gomez, C. A. SilveraBatista, and M. Rafat. Growth and characterization of irradiated organoids from mammary glands. J. Vis. Exp. 147:e59293, 2019.
Hama, H., H. Hioki, K. Namiki, T. Hoshida, H. Kurokawa, F. Ishidate, T. Kaneko, T. Akagi, T. Saito, T. Saido, and A. Miyawaki. ScaleS: an optical clearing palette for biological imaging. Nat. Neurosci. 18:1518–1529, 2015.
Han, Y. L., P. Ronceray, G. Xu, A. Malandrino, R. D. Kamm, M. Lenz, C. P. Broedersz, and M. Guo. Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc. Natl. Acad. Sci. 115:4075–4080, 2018.
Hanahan, D., and R. A. Weinberg. Hallmarks of cancer: the next generation. Cell 144:646–674, 2011.
Huang, Q., F. Li, X. Liu, W. Li, W. Shi, F. F. Liu, B. O’Sullivan, Z. He, Y. Peng, A. C. Tan, L. Zhou, J. Shen, G. Han, X. J. Wang, J. Thorburn, A. Thorburn, A. Jimeno, D. Raben, J. S. Bedford, and C. Y. Li. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 17:860–866, 2011.
Huang, X., F. Yuan, M. Liang, H. W. Lo, M. L. Shinohara, C. Robertson, and P. Zhong. M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS ONE 7:1–11, 2012.
Huebner, R. J., N. M. Neumann, and A. J. Ewald. Mammary epithelial tubes elongate through MAPK-dependent coordination of cell migration. Development 143:983–995, 2016.
Hwang, P. Y., A. Brenot, A. C. King, G. D. Longmore, and S. C. George. Randomly distributed K14+ breast tumor cells polarize to the leading edge and guide collective migration in response to chemical and mechanical environmental cues. Cancer Res. 79:1899–1912, 2019.
Jadaliha, M., O. Gholamalamdari, W. Tang, Y. Zhang, A. Petracovici, Q. Hao, A. Tariq, T. G. Kim, S. E. Holton, D. K. Singh, X. L. Li, S. M. Freier, S. Ambs, R. Bhargava, A. Lal, S. G. Prasanth, J. Ma, and K. V. Prasanth. A natural antisense lncRNA controls breast cancer progression by promoting tumor suppressor gene mRNA stability. PLoS Genet. 14:e1007802, 2018.
Jalili-Firoozinezhad, S., R. Prantil-Baun, A. Jiang, R. Potla, T. Mammoto, J. C. Weaver, T. C. Ferrante, H. J. Kim, J. M. S. Cabral, O. Levy, and D. E. Ingber. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 9:1–14, 2018.
Jamieson, P. R., J. F. Dekkers, A. C. Rios, N. Y. Fu, G. J. Lindeman, and J. E. Visvader. Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144:1065–1071, 2016.
Jardé, T., B. Lloyd-Lewis, M. Thomas, H. Kendrick, L. Melchor, L. Bougaret, P. D. Watson, K. Ewan, M. J. Smalley, and T. C. Dale. Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat. Commun. 7:1–14, 2016.
Jiang, Y., T. Verbiest, A. M. Devery, S. M. Bokobza, A. M. Weber, K. B. Leszczynska, E. M. Hammond, and A. J. Ryan. Hypoxia potentiates the radiation-sensitizing effect of olaparib in human non-small cell lung cancer xenografts by contextual synthetic lethality. Int. J. Radiat. Oncol. Biol. Phys. 95:772–781, 2016.
Ke, M. T., S. Fujimoto, and T. Imai. SeeDB: A simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 16:1154–1161, 2013.
Kim, M. Y., T. Oskarsson, S. Acharyya, D. X. Nguyen, X. H. F. Zhang, L. Norton, and J. Massagué. Tumor self-seeding by circulating cancer cells. Cell 139:1315–1326, 2009.
Kioi, M., J. M. Brown, H. Vogel, G. Schultz, R. M. Hoffman, and G. R. Harsh. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 120:694–705, 2010.
Klingelhutz, A. J., F. A. Gourronc, A. Chaly, D. A. Wadkins, A. J. Burand, K. R. Markan, S. O. Idiga, M. Wu, M. J. Potthoff, and J. A. Ankrum. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci. Rep. 8:1–12, 2018.
Koledova, Z. 3D coculture of mammary organoids with fibrospheres: a model for studying epithelial–stromal interactions during mammary branching morphogenesis. In: Methods in Molecular Biology, edited by Z. Koledova. New York, NY: Humana Press, 2017.
Kopanska, K. S., Y. Alcheikh, R. Staneva, D. Vignjevic, and T. Betz. Tensile forces originating from cancer spheroids facilitate tumor invasion. PLoS ONE 11:1–23, 2016.
Kuen, J., D. Darowski, T. Kluge, and M. Majety. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS One 12:1–19, 2017.
LaBarge, M. A., J. C. Garbe, and M. R. Stampfer. Processing of human reduction mammoplasty and mastectomy tissues for cell culture. J. Vis. Exp. 71:e50011, 2013.
Laperrousaz, B., S. Porte, S. Gerbaud, V. Härmä, F. Kermarrec, V. Hourtane, F. Bottausci, X. Gidrol, and N. Picollet-D’Hahan. Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens. Nucleic Acids Res. 46:1–13, 2018.
Lasfargues, E. Cultivation and behavior in vitro of the normal mammary epithelium of the adult mouse. Anat. Rec. 127:117–129, 1957.
Linde, N., M. Casanova-Acebes, M. S. Sosa, A. Mortha, A. Rahman, E. Farias, K. Harper, E. Tardio, I. ReyesTorres, J. Jones, J. Condeelis, M. Merad, and J. A. Aguirre-Ghiso. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 9:1–14, 2018.
Linnemann, J. R., H. Miura, L. K. Meixner, M. Irmler, U. J. Kloos, B. Hirschi, H. S. Bartsch, S. Sass, J. Beckers, F. J. Theis, C. Gabka, K. Sotlar, and C. H. Scheel. Quantification of regenerative potential in primary human mammary epithelial cells. Development 142:3239–3251, 2015.
Liu, H. L., H. Y. Hsieh, L. A. Lu, C. W. Kang, M. F. Wu, and C. Y. Lin. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J. Transl. Med. 10:1–14, 2012.
Loi, S., S. Michiels, R. Salgado, N. Sirtaine, V. Jose, D. Fumagalli, P. L. Kellokumpu-Lehtinen, P. Bono, V. Kataja, C. Desmedt, M. J. Piccart, S. Loibl, C. Denkert, M. J. Smyth, H. Joensuu, and C. Sotiriou. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann. Oncol. 25:1544–1550, 2014.
López Alfonso, J. C., S. Parsai, N. Joshi, A. Godley, C. Shah, S. A. Koyfman, J. J. Caudell, C. D. Fuller, H. Enderling, and J. G. Scott. Temporally feathered intensity-modulated radiation therapy: a planning technique to reduce normal tissue toxicity. Med. Phys. 45:3466–3474, 2018.
Lowery, A., M. Kell, R. Glynn, M. Kerin, and K. Sweeney. Locoregional recurrence after breast cancer surgery : a systematic review by receptor phenotype. Breast Cancer Res. Treat. 133:831–841, 2012.
Lu, L., M. Jiang, C. Zhu, J. He, and S. Fan. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3′-Diindolylmethane (DIM). Free Radic. Biol. Med. 130:244–255, 2019.
Malandrino, A., M. Mak, R. D. Kamm, and E. Moeendarbary. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extrem. Mech. Lett. 21:25–34, 2018.
Malandrino, A., X. Trepat, R. D. Kamm, and M. Mak. Dynamic filopodial forces induce accumulation, damage, and plastic remodeling of 3D extracellular matrices. PLoS Comput. Biol. 15:1–26, 2019.
Martin, I., D. Wendt, and M. Heberer. The role of bioreactors in tissue engineering. Trends Biotechnol. 22:80–86, 2004.
Meng, L., Y. Cheng, X. Tong, S. Gan, Y. Ding, Y. Zhang, C. Wang, L. Xu, Y. Zhu, J. Wu, Y. Hu, and A. Yuan. Tumor oxygenation and hypoxia inducible factor-1 functional inhibition via a reactive oxygen species responsive nanoplatform for enhancing radiation therapy and abscopal effects. ACS Nano 12:8308–8322, 2018.
Metcalfe, C., N. M. Kljavin, R. Ybarra, and F. J. De Sauvage. Lgr5+stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14:149–159, 2014.
Miller, D. H., D. X. Jin, E. S. Sokol, J. R. Cabrera, D. A. Superville, R. A. Gorelov, C. Kuperwasser, and P. B. Gupta. BCL11B drives human mammary stem cell self-renewal in vitro by inhibiting basal differentiation. Stem Cell Rep. 10:1131–1145, 2018.
Miller, J. S., K. R. Stevens, M. T. Yang, B. M. Baker, D. H. T. Nguyen, D. M. Cohen, E. Toro, A. A. Chen, P. A. Galie, X. Yu, R. Chaturvedi, S. N. Bhatia, and C. S. Chen. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11:768–774, 2012.
Montay-Gruel, P., A. Bouchet, M. Jaccard, D. Patin, R. Serduc, W. Aim, K. Petersson, B. Petit, C. Bailat, J. Bourhis, E. Bräuer-Krisch, and M. C. Vozenin. X-rays can trigger the FLASH effect: ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 129:582–588, 2018.
Montay-Gruel, P., K. Petersson, M. Jaccard, G. Boivin, J. F. Germond, B. Petit, R. Doenlen, V. Favaudon, F. Bochud, C. Bailat, J. Bourhis, and M. C. Vozenin. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 124:365–369, 2017.
Morgan, M. M., M. K. Livingston, J. W. Warrick, E. M. Stanek, E. T. Alarid, D. J. Beebe, and B. P. Johnson. Mammary fibroblasts reduce apoptosis and speed estrogen-induced hyperplasia in an organotypic MCF7-derived duct model. Sci. Rep. 8:1–13, 2018.
Morini, J., G. Babini, S. Barbieri, G. Baiocco, and A. Ottolenghi. The interplay between radioresistant Caco-2 cells and the immune system increases epithelial layer permeability and alters signaling protein spectrum. Front. Immunol. 8:1–12, 2017.
Nagle, P. W., N. A. Hosper, L. Barazzuol, A. L. Jellema, M. Baanstra, M. J. Van Goethem, S. Brandenburg, U. Giesen, J. A. Langendijk, P. Van Luijk, and R. P. Coppes. Lack of DNA damage response at low radiation doses in adult stem cells contributes to organ dysfunction. Clin. Cancer Res. 24:6583–6593, 2018.
Nguyen, D. H., H. A. Oketch-Rabah, I. Illa-Bochaca, F. C. Geyer, J. S. Reis-Filho, J. H. Mao, S. A. Ravani, J. Zavadil, A. D. Borowsky, D. J. Jerry, K. A. Dunphy, J. H. Seo, S. Haslam, D. Medina, and M. H. Barcellos-Hoff. Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell 19:640–651, 2011.
Nguyen-Ngoc, K.-V., K. J. Cheung, A. Brenot, E. R. Shamir, R. S. Gray, W. C. Hines, P. Yaswen, Z. Werb, and A. J. Ewald. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. 89:E2595–E2604, 2012.
Nguyen-Ngoc, K.-V., E. R. Shamir, R. J. Huebner, J. N. Beck, K. J. Cheung, and A. J. Ewald. 3D culture assays of murine mammary branching morphogenesis and epithelial invasion. In: Tissue Morphogenesis: Methods and Protocols, edited by C. M. Nelson. New York: Springer, 2015, pp. 135–162.
Noël, G., C. Godon, M. Fernet, N. Giocanti, F. Mégnin-Chanet, and V. Favaudon. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol. Cancer Ther. 5:564–574, 2006.
Nozaki, K., W. Mochizuki, Y. Matsumoto, T. Matsumoto, M. Fukuda, T. Mizutani, M. Watanabe, and T. Nakamura. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol. 51:206–213, 2016.
Ohuchida, K., K. Mizumoto, M. Murakami, L. W. Qian, N. Sato, E. Nagai, K. Matsumoto, T. Nakamura, and M. Tanaka. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 64:3215–3222, 2004.
Panciera, T., L. Azzolin, A. Fujimura, D. Di Biagio, C. Frasson, S. Bresolin, S. Soligo, G. Basso, S. Bicciato, A. Rosato, M. Cordenonsi, and S. Piccolo. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19:725–737, 2016.
Paris, F., Z. Fuks, A. Kang, P. Capodieci, G. Juan, D. Ehlelter, A. Halmovitz-Friedman, C. Cordon-Cardo, and R. Kolesnick. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293:293–297, 2001.
Poglio, S., S. Galvani, S. Bour, M. André, B. Prunet-Marcassus, L. Pénicaud, L. Casteilla, and B. Cousin. Adipose tissue sensitivity to radiation exposure. Am. J. Pathol. 174:44–53, 2009.
Powell, D. R., and A. Huttenlocher. Neutrophils in the tumor microenvironment. Trends Immunol. 37:41–52, 2016.
Rafat, M., T. A. Aguilera, M. Vilalta, L. L. Bronsart, L. A. Soto, R. von Eyben, M. A. Golla, Y. Ahrari, S. Melemenidis, A. Afghahi, M. J. Jenkins, A. W. Kurian, K. C. Horst, A. J. Giaccia, and E. E. Graves. Macrophages promote circulating tumor cell–mediated local recurrence following radiotherapy in immunosuppressed patients. Cancer Res. 78:4241–4252, 2018.
Ray, G., S. Batra, N. K. Shukla, S. Deo, V. Raina, S. Ashok, and S. A. Husain. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res. Treat. 59:163–170, 2000.
Rodemann, H. P., and M. A. Blaese. Responses of normal cells to ionizing radiation. Semin. Radiat. Oncol. 17:81–88, 2007.
Rodney Withers, H. Biological basis of radiation therapy for cancer. Lancet 339:156–159, 1992.
Rossi, G., A. Manfrin, and M. P. Lutolf. Progress and potential in organoid research. Nat. Rev. Genet. 19:671–687, 2018.
Sachs, N., J. de Ligt, O. Kopper, E. Gogola, G. Bounova, F. Weeber, A. V. Balgobind, K. Wind, A. Gracanin, H. Begthel, J. Korving, R. van Boxtel, A. A. Duarte, D. Lelieveld, A. van Hoeck, R. F. Ernst, F. Blokzijl, I. J. Nijman, M. Hoogstraat, M. van de Ven, D. A. Egan, V. Zinzalla, J. Moll, S. F. Boj, E. E. Voest, L. Wessels, P. J. van Diest, S. Rottenberg, R. G. J. Vries, E. Cuppen, and H. Clevers. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172:373–386, 2018.
Sato, T., and H. Clevers. SnapShot: Growing organoids from stem cells. Cell 161:1700–1700.e1, 2015.
Sato, T., D. E. Stange, M. Ferrante, R. G. J. Vries, J. H. Van Es, S. Van Den Brink, W. J. Van Houdt, A. Pronk, J. Van Gorp, P. D. Siersema, and H. Clevers. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772, 2011.
Sayarath, M., M.-C. Vozenin, L. Caplier, J. Bourhis, C. Fouillade, P. Hupe, J. Hall, V. Favaudon, M.-F. Poupon, V. Monceau, J.-J. Fontaine, F. Pouzoulet, and I. Brito. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 6:1–9, 2014.
Schaue, D., E. L. Kachikwu, and W. H. McBride. Cytokines in radiobiological responses: a review. Radiat. Res. 178:505–523, 2012.
Senra, J. M., B. A. Telfer, K. E. Cherry, C. M. McCrudden, D. G. Hirst, M. J. O’Connor, S. R. Wedge, and I. J. Stratford. Inhibition of PARP-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol. Cancer Ther. 10:1949–1958, 2011.
Shamir, E. R., E. Pappalardo, D. M. Jorgens, K. Coutinho, W. T. Tsai, K. Aziz, M. Auer, P. T. Tran, J. S. Bader, and A. J. Ewald. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol. 204:839–856, 2014.
Sherry, A. D., R. von Eyben, N. B. Newman, P. Gutkin, I. Mayer, K. Horst, A. B. Chakravarthy, and M. Rafat. Systemic inflammation after radiation predicts locoregional recurrence, progression, and mortality in stage II-III triple-negative breast cancer. Int. J. Radiat. Oncol. 2019. https://doi.org/10.1016/j/ijrobp.2019.11.398.
Siegel, R. L., K. D. Miller, and A. Jemal. Cancer statistics, 2018. Cancer J. Clin. 69:7–34, 2019.
Simmons, D. A., F. M. Lartey, E. Schüler, M. Rafat, G. King, A. Kim, R. Ko, S. Semaan, S. Gonzalez, M. Jenkins, P. Pradhan, Z. Shih, J. Wang, R. von Eyben, E. E. Graves, P. G. Maxim, F. M. Longo, and B. W. Loo. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 139:4–10, 2019.
Sirka, O. K., E. R. Shamir, and A. J. Ewald. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J. Cell Biol. 217:3368–3381, 2018.
Skrzydlewska, E., S. Sulkowksi, M. Koda, B. Zalewski, L. Kanczuga-Koda, and M. Sulkowska. Lipid peroxidation and antioxidant status in colorecal cancer. World J Gastroenterol. 11:403–406, 2005.
Slonina, D., B. Biesaga, K. Urbanski, and Z. Kojs. Low-dose radiation response of primary keratinocytes and fibroblasts from patients with cervix cancer. Radiat. Res. 167:251–259, 2007.
Sofia Vala, I., L. R. Martins, N. Imaizumi, R. J. Nunes, J. Rino, F. Kuonen, L. M. Carvalho, C. Rüegg, I. M. Grillo, J. T. Barata, M. Mareel, and S. C. R. Santos. Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis. PLoS ONE 5:1–13, 2010.
Spill, F., C. Bakal, and M. Mak. Mechanical and systems biology of cancer. Comput. Struct. Biotechnol. J. 16:237–245, 2018.
Stephens, T. C., G. A. Currie, and J. H. Peacock. Repopulation of gamma-irradiated lewis lung carcinoma by malignant cells and host macrophage progenitors. Br. J. Cancer 38:573–582, 1978.
Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int. J. Radiat. Biol. 91:1–12, 2015.
Taniguchi, C. M., Y. R. Miao, A. N. Diep, C. Wu, E. B. Rankin, T. F. Atwood, L. Xing, and A. J. Giaccia. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl. Med. 6:1–9, 2014.
Tevis, K. M., R. J. Cecchi, Y. L. Colson, and M. W. Grinstaff. Mimicking the tumor microenvironment to regulate macrophage phenotype and assessing chemotherapeutic efficacy in embedded cancer cell/macrophage spheroid models. Acta Biomater. 50:271–279, 2017.
Tsai, K. K. C., E. Y. Y. Chuang, J. B. Little, and Z. M. Yuan. Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res. 65:6734–6744, 2005.
Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 524:13–30, 2017.
Unver, N., and F. Mcallister. IL-6 family cytokines: key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 41:10–17, 2018.
van Luijk, P., S. Pringle, S. Brandenburg, V. V. Moiseenko, H. P. van der Laan, R. P. Coppes, J. Wu, J. A. Langendijk, M. J. Witjes, J. M. Schippers, J. O. Deasy, A. Hovan, A. van der Schaaf, M. Baanstra, H. Faber, and R. G. J. Kierkels. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci. Transl. Med. 7:1–8, 2015.
Vanpouille-Box, C., A. Alard, M. J. Aryankalayil, Y. Sarfraz, J. M. Diamond, R. J. Schneider, G. Inghirami, C. N. Coleman, S. C. Formenti, and S. Demaria. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8:1–15, 2017.
Venkatesulu, B. P., L. S. Mahadevan, M. L. Aliru, X. Yang, M. H. Bodd, P. K. Singh, S. W. Yusuf, J. Abe, and S. Krishnan. Radiation-induced endothelial vascular injury. JACC Basic Transl. Sci. 3:563–572, 2018.
Vilalta, M., M. Rafat, and E. E. Graves. Effects of radiation on metastasis and tumor cell migration. Cell. Mol. Life Sci. 73:2999–3007, 2016.
von Essen, C. F. Radiation enhancement of metastasis: a review. Clin. Exp. Metastasis 9:77–104, 1991.
Wang, X. Y., Z. H. Jin, B. W. Gan, S. W. Lv, M. Xie, and W. H. Huang. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip 14:2709–2716, 2014.
Wang, X., Q. Sun, and J. Pei. Microfluidic-based 3D engineered microvascular networks and their applications in vascularized microtumor models. Micromachines 9:1–26, 2018.
Wang, F., V. M. Weaver, O. W. Petersen, C. A. Larabell, S. Dedhar, P. Briand, R. Lupu, and M. J. Bissell. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. 95:14821–14826, 1998.
Weigelt, B., J. L. Peterse, and L. J. Van’t Veer. Breast cancer metastasis: markers and models. Nat. Rev. Cancer 5:591–602, 2005.
Welsch, C. W. Review of the effects of dietary fat on experimental mammary gland tumorigenesis: role of lipid peroxidation. Free Radic. Biol. Med. 18:757–773, 1995.
Winslow, S., A. Scholz, P. Rappl, T. F. Brauß, C. Mertens, M. Jung, A. Weigert, B. Brüne, and T. Schmid. Macrophages attenuate the transcription of CYP1A1 in breast tumor cells and enhance their proliferation. PLoS ONE 14:1–12, 2019.
Wong, E., S. Chaudhry, and M. Rossi. Breast cancer. McMaster Pathophysiol. Rev., 2012.
Wouters, B. G. Oncology scan-how radiation effects on cells in the stromal microenvironment influence tumor development, proliferation, and recovery. Int. J. Radiat. Oncol. Biol. Phys. 86:593–595, 2013.
Xu, K., and R. J. Buchsbaum. Isolation of mammary epithelial cells from three-dimensional mixed-cell spheroid co-culture. J. Vis. Exp. 62:e3760, 2012.
Zanoni, M., F. Piccinini, C. Arienti, A. Zamagni, S. Santi, R. Polico, A. Bevilacqua, and A. Tesei. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 6:1–11, 2016.
Zhang, Z., F. Jin, X. Lian, M. Li, G. Wang, B. Lan, H. He, G. D. Liu, Y. Wu, G. Sun, C. X. Xu, and Z. Z. Yang. Genistein promotes ionizing radiation-induced cell death by reducing cytoplasmic Bcl-xL levels in non-small cell lung cancer. Sci. Rep. 8:1–9, 2018.
Zhang, C., S. Wang, H. P. Israel, S. X. Yan, D. P. Horowitz, S. Crockford, D. Gidea-Addeo, K. S. Clifford Chao, K. Kalinsky, and E. P. Connolly. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. Springerplus 4:1–9, 2015.
Zhao, J., M. Griffin, J. Cai, S. Li, P. E. M. Bulter, and D. M. Kalaskar. Bioreactors for tissue engineering: an update. Biochem. Eng. J. 109:268–281, 2016.
Zhong, J., S. A. Krawczyk, R. Chaerkady, H. Huang, R. Goel, J. S. Bader, G. W. Wong, B. E. Corkey, and A. Pandey. Temporal profiling of the secretome during adipogenesis in humans. J. Proteome Res. 9:5228–5238, 2010.
Zubeldia-Plazaola, A., L. Recalde-Percaz, N. Moragas, M. Alcaraz, X. Chen, M. Mancino, P. Fernández-Nogueira, M. P. de Puig, F. Guzman, A. Noguera-Castells, A. López-Plana, E. Enreig, N. Carbó, V. Almendro, P. Gascón, P. Bragado, and G. Fuster. Glucocorticoids promote transition of ductal carcinoma in situ to invasive ductal carcinoma by inducing myoepithelial cell apoptosis. Breast Cancer Res. 20:1–16, 2018.
Acknowledgments
This work was financially supported by NIH Grant #R00CA201304.
Conflict of interest
Benjamin C. Hacker and Marjan Rafat declare that they have no conflicts of interest.
Research Involving Human Rights
No human studies were carried out by the authors of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacker, B.C., Rafat, M. Organoids as Complex In Vitro Models for Studying Radiation-Induced Cell Recruitment. Cel. Mol. Bioeng. 13, 341–357 (2020). https://doi.org/10.1007/s12195-020-00625-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-020-00625-0