Metabolic and Population Effects of Winter Tick Infestations on Moose: Unique Evolutionary Circumstances?
- Department of Natural Resources and the Environment, University of New Hampshire, Durham, NH, United States
Moose (Alces alces) have evolved to store adequate body fat to emerge from winter in adequate nutritional condition that is key to annual productivity and neonatal survival. Blood consumption by winter ticks (Dermacentor albipictus) affects survival and productivity of moose, often resulting in marked local and regional die-offs of calves. Concurrent with an unprecedented frequency of winter tick epizootics (>50% calf mortality) in the northeastern United States, productivity but not mortality of adult female moose also has declined because of low rates of twinning and calving. Chronic blood loss to winter ticks in late winter-early spring negatively affects pregnant cows in their energy- and protein-costly 3rd trimester of pregnancy that will eventually calve and lactate initially in an environment low in digestible energy and protein. To describe this dynamic, I calculated the endogenous fat balance of different-sized pregnant cows by developing energy-balance equations that accounted temporally for gestation, winter tick infestation, and lactation under two consumption levels. The analysis revealed the critical importance of body mass and body fat as only large cows (25% pre-winter body fat) were immune from depletion of body fat at birth in all scenarios. Mid-sized cows (20% body fat) depleted fat reserves during gestation in most scenarios, and small cows (15% body fat) in all scenarios. The infestation and forage- consumption levels influenced the predicted date of fat depletion up to several weeks, and failed calving or mortal mass loss associated with rapid loss of endogenous protein was possible in mid-sized and small cows. The continual decline in demographic parameters points to reduced body mass and body fat over time, or increased numbers of mid-sized and small cows in the population with lower reproductive potential. This regional population is confronted with a unique and sustained combination of environmental and parasitic conditions associated with a warming climate that markedly affects its survival and reproduction in quality habitat, a unique occurrence in their evolutionary history.
Introduction
Nutritional carrying capacity of a wildlife population is typically described relative to resource availability and environmental constraints that limit that availability. In seasonal environments, ungulates balance demand and constraints relative to reproductive success (Parker et al., 2009). Although considered a “southern range” population, moose in the northeastern United States (hereafter Northeast) occupy a seasonal environment with a relatively long winter and short growing season that constrain resource availability—spring green-up typically occurs in late May-early June and leaf senescence in late September-early October. As with other moose populations, winter forage intake is inadequate to maintain body mass throughout winter (Schwartz and Renecker, 2007), and pregnant cows store and subsequently catabolize endogenous fat reserves and protein to meet the energy and protein requirements of maintenance, gestation, and lactation (Parker et al., 2009). Throughout most moose range in North America and specifically in the Northeast, the entirety of gestation and the initial 1–2 weeks of lactation occur prior to spring green-up after which forage increases in digestible protein and energy. It follows that adult cows have evolved to survive winter with adequate tissue resources to provide for the energy- and protein-costly last trimester of gestation and early lactation, and that late-winter condition and adequate fetal growth during the last trimester of gestation are related directly to neonatal survival (Keech et al., 2000; Parker et al., 2009); effectively, they are capital breeders that rely on body reserves to produce successfully.
The moose population in the Northeast United States (Maine, New Hampshire, and Vermont) irrupted during the late 1970s through early 1990s in response to extensive forest harvesting associated with a severe spruce budworm (Choristoneura fumiferana) epidemic (Bontaites and Gustafson, 1993; Dunfey-Ball, 2017). Although in slow decline for the past 10–15 years, it still remains the largest contiguous regional population in the lower 48 states, exceeding >50,000 animals. This decline spurred regional research and since 2014 has included >500 radio-marked cows and calves to measure pregnancy rate, calf production and survival, adult survival, and successive birthing rate of adult cows (Jones et al., 2017, 2019; Ellingwood et al., 2019). Annual measurements of harvested animals revealed that body mass of adult animals was trending down and low body mass was limiting sexual maturation of yearling cows (Adams and Pekins, 1995; Musante et al., 2010; Bergeron et al., 2013).
Current research indicates that the birthing rate of adult cows is measurably lower than the pregnancy rate, twinning is rare, and yearlings rarely breed in northern New Hampshire and western Maine (Jones et al., 2019; Powers, 2019). Further, epizootic-level calf mortality (>50%; 10-month old calves) in late March–April because of infestation of winter ticks (Dermacentor albipictus) is occurring with unprecedented frequency and regardless of winter severity (Ellingwood, 2018; Jones et al., 2019; Powers, 2019). Evidence that the nutritional condition of moose is not compromised by habitat quality includes: (1) forest regeneration is not affected by over-browsing (Bergeron et al., 2011; Andreozzi et al., 2014); timber harvesting creates abundant and stable optimal foraging habitat (Dunfey-Ball, 2017); and no starvation was documented with radio-marked animals in 5 years of study (Ellingwood, 2018; Jones et al., 2019; Powers, 2019). Rather, the population dynamics of much of this regional population reflects the annual and continual (5 in 6 years from 2014 to 2019) influence of parasitism by winter ticks (Ellingwood et al., 2020).
The severity of winter tick infestation is typically a function of 3 factors—moose (host) density and favorable environmental conditions for larval winter ticks (Samuel, 2007), and overlap of seasonal habitat use (Healy et al., 2018)—that manifest in severe winters that simultaneously reduce animal condition and increase the relative impact of infestations (Samuel, 2007). In contrast, the moose population in the Northeast has not experienced increased length or severity of winter in recent epizootic years. Rather, later starting winters associated with climate change are considered a unique ecological influence that extends the autumnal questing period for larval winter ticks (Jones et al., 2019; Healy et al., 2020). This longer questing period alters the direct relationship between host and parasite density, or tick abundance and infestation, while largely negating the influence of winter severity during epizootic years.
Earlier analyses identified a stark contrast between predicted calf versus adult mortality at severe infestation levels (Musante et al., 2007), and field studies corroborate that winter ticks rarely cause adult mortality in the Northeast (Jones et al., 2019) or elsewhere (Samuel, 2007). Nonetheless, these analyses also demonstrated that severe infestation by ticks measurably impacted the energy and protein balance of adult cows (Musante et al., 2007). Because productivity is declining in the Northeast (Jones et al., 2019), this analysis was designed to measure the temporal influence of winter ticks on energy and protein balance of pregnant cows to identify the potential effect of infestation on productivity in the current year, and to better understand and predict the sustained impact of high infestations. Multiple factors including body mass and composition, infestation level of winter ticks (infestation), and forage consumption were varied in energy-balance equations to illustrate a range of potential outcomes in body condition. I hypothesized that: (1) fat reserves of heavier cows would provide a buffer during the last trimester of gestation and early lactation against the energy-protein losses associated with high infestation; (2) small and mid-sized cows would lose endogenous protein (muscle mass) rapidly during the 3rd trimester of gestation at high infestation; (3) all cows would be in reasonable physical condition at birth at low infestation by ticks; and (4) low forage consumption would only influence condition at high infestation.
Materials and Methods
The empirical data used in the analyses were collected from radio-marked moose studied in northern New Hampshire in 2014–2018 (Jones et al., 2017, 2019; Ellingwood, 2018; Powers, 2019). Animal capture and handling protocols were approved by the Institutional Animal Care and Use Committee at the University of New Hampshire (IACUC #130805). These moose occupied contiguous, commercial forestland that is a transition of the northern hardwood and boreal forest types; a thorough description is found in Jones et al. (2019). Importantly, no predator of moose exists in the study area except black bears (Ursus americanus) that are considered a minor predator of neonates; calf survival is high (∼70%) 60-days post-birth (Musante et al., 2010; Jones et al., 2017). The current regional moose density is estimated as 0.46 moose/km2 (0.87 moose/km2 in 2005), but the local density within the study area (∼1,250 km2; Jones et al., 2019) is considered higher; e.g., 45–50 cows and calves combined were captured annually within this area for 5 consecutive winters (January 2014–2018). Annual adult survival averaged 83% in 2014–2019 (Powers, 2019), with calf mortality concentrated in late March–April and associated with winter tick infestation (Jones et al., 2017, 2019); epizootics (61–77% mortality) documented with radio-marked calves occurred in 2014, 2015, 2016, and 2018 (Powers, 2019).
Energy balance equations were used to track cow condition (body mass and fat level) from 10 January to 23 May, a period that encompassed the second and third trimesters of gestation, feeding (blood removal) by nymphs and adult winter ticks, and 1 week of lactation. The field metabolic rate (FMR) was initially estimated as a multiple of the maintenance energy cost of a non-pregnant cow, and elevated with the estimated costs of gestation, lactation, and blood loss (replacement cost) from winter ticks; these costs were balanced against forage consumption and endogenous reserves to maintain body mass. Each variable was set relative to empirical measurements in the environment, or if a single value, to the benefit of the experimental moose.
Field Metabolic Rate (FMR)
The FMR of an adult cow was set as 1.1 times the maintenance energy requirement [603 kJ/kg body mass (BM)0.75/d] as measured directly in forage consumption trials (Schwartz et al., 1988b). This value is equivalent to 1.7 × the fasting metabolic rate of moose measured by Regelin et al. (1985) as well as the average FMR measured in winter with doubly labeled water with both captive adult female white-tailed deer (Odocoileus virginianus) living in a 1.5 ha pen (Eckert, 2004) and free-ranging adult white-tailed deer (Pekins, 1995) that demonstrated energy-conserving behavior. In general, annual FMR of free-ranging animals is assumed to be ∼2 × basal metabolic rate (Robbins, 1993). The winter FMR measured with doubly labeled water averaged ∼2.1 × basal metabolic rate in free-ranging white-tailed deer fawns (Tarr and Pekins, 2002) expected to operate at higher metabolism than adults, and adult female black-tailed deer (Odocoileus hemonius sitchensis; Parker et al., 1999).
Body Mass (BM)
Body mass was expressed as low (325 kg), mid-sized (350 kg), and high (375 kg); these successive values differed by 5% to reflect a similar percentage difference in body fat and represent a reasonable range of winter condition (Schwartz and Renecker, 2007). I assumed that BM (including the fetus) was maintained throughout gestation and was then lowered 18 kg post-birth during the first week of lactation. This presumed a birth mass of 15 kg (Schwartz, 2007) and that the fetus represents 82% of the estimated energy deposition associated with the fetus and placenta combined (Oftedal, 1985).
Body Fat (BF)
Body fat was set at 15% (49 kg), 20% (70 kg), and 25% (94 kg) to correspond proportionally with the low, mid-sized, and high BM, respectively; at the start of winter, adult moose typically have 20–26% BF (Schwartz et al., 1988a; Schwartz and Renecker, 2007). These proportions were assumed as the initial% BF in January and converted to an energy equivalent (39.33 kJ/g) as required to meet energy balance. If BF was exhausted during gestation, endogenous protein (BP, muscle mass) was catabolized and converted with an energy equivalent (16.74 kJ/g).
Gestation
The length of gestation was assumed to be 231 days, which set the 3rd trimester at 77 days (01 March to 16 May); the annual median date of birth was 16–18 May in the study area (Jones et al., 2017). Moose delay most (90%) fetal growth (Schwartz and Hundertmark, 1993; Schwartz, 2007) until the 3rd trimester so that proportional cost was assigned to that period; 10% of the cost was assigned to the 48-day portion of the 2nd trimester from early January through February.
The peak cost of gestation is 1.8–1.9 times higher than that of non-pregnant animals with cost rising exponentially in the 3rd trimester (Schwartz and Hundertmark, 1993; Pekins et al., 1998; Schwartz, 2007). To account for the increasing rate, the 3rd trimester was broken into three periods of 25, 26, and 26 days that were assigned a cost of 1.2, 1.4, and 1.7 times the fasting metabolic rate (355.6 kJ/kgBM0.75/d; Regelin et al., 1985). These rates represent the midpoint multiplier in each period based on the predictive equation for white-tailed deer (Pekins et al., 1998). Further, these three periods are representative stages of blood loss from adult winter ticks (see below).
Lactation
Energy cost of lactation was estimated for 7 days post-birth (16–23 May) during which daily forage consumption was assumed equal to the winter diet, or diet prior to spring green-up. The energy cost of lactation was set at 23,800 kJ/d for each cow as calculated from three factors: (1) an average energy daily intake of 1,820 kJ/kg0.75/d by calves in their first 30 days (Reese and Robbins, 1994); (2) the energetic efficiency of milk production is 65% (Oftedal, 1985; Schwartz and Renecker, 2007); and (3) calf BM averaged 17.5 kg during the 7-day period (785 g daily increase from 15 kg birth weight; Reese and Robbins, 1994). I recognized that spring green-up could occur >1 week post-partum, or conversely, that nutritional quality of forage improves as stem and bud chemistry responds to warming prior to leaf out.
Forage Consumption
Overall, intake and forage quality were set at the upper end of values for moose. Daily forage consumption was set at two levels: 1 and 1.2% BM were used to simulate consumption at high intake or habitat quality (Schwartz and Renecker, 2007). These values correspond to multiple measurements and estimates of winter forage intake by moose, and based on a compilation of multiple studies; Schwartz and Renecker (2007) estimated that the metabolizable energy of a mixed woody browse diet as 9.2 kJ/dry g on good winter habitat (upper end of value). Although diet quality declines through winter as browse is removed, this value was used throughout the experimental period because local studies indicated that the study area contained plentiful optimal foraging habitat (Bergeron et al., 2011; Dunfey-Ball, 2017). For comparison, the 1% level was defined as low and the 1.2% level as high consumption; however, neither value is considered low on a continuum of intake.
Infestation Level
Infestation was set at two levels that represent moderate and severe infestations (30,000 and 90,000 winter ticks); these values were near the extremes (∼20,000–95,000) of infestation measured on whole hides of dead calves in the study area (Jones et al., 2019). Adult female ticks are the primary cause of blood loss in March–April and are assumed to represent ∼25% of ticks on moose (Samuel, 2004; Musante et al., 2007); however, recent measurements indicated a 50:50 sex ratio on calf hides in New Hampshire (unpublished data, Pekins). Therefore, both ratios (25 and 50% female) were used to estimate blood loss.
Blood loss was conservatively estimated as 0.5 g/engorged adult female tick in previous studies (Samuel, 2004; Musante et al., 2007) despite higher estimates of 0.6 and 0.85 g (Glines, 1983; Addison et al., 1998); the conservative value was used to account for tick loss from grooming. Nevertheless, because infestation level was measured directly on dead calves in New Hampshire (Jones et al., 2017, 2019), I used 0.75 g, which is within the range of the higher estimates. Further, these weights were multiplied by 2.5 to account for the total blood loss associated with feeding which is estimated as 2–3 × the engorged tick mass (Sonenshine, 1991). A conservative estimate of 1 g = 1 mL of blood was used to estimate blood volume loss with a replacement energy cost of 4.81 kJ/mL (Musante et al., 2007).
Engorgement by adult ticks occurs over an 8-week period and is concentrated in the middle 4 weeks of infestation (Drew and Samuel, 1989; Samuel, 2004). Therefore, proportional blood loss was established in three distinct periods of 2, 4, and 2 weeks: 01 March–15 March (10%); 16 March–15 April (80%); and 16 April–1 May (10%)—tick drop-off and calf mortality (Jones et al., 2019) are concentrated in the middle 4-week period.
Previous studies disregarded blood loss associated with nymphs and engorged adult male ticks because of the size differential relative to an engorged adult female. Feeding by nymphs occurs over >2 months in December–February (Addison et al., 1998; Samuel, 2004), effectively minimizing the daily energetic impact to replace blood loss. In contrast, adult males remove blood simultaneously with adult females during the 8-week engorgement period. Because the average weight of an engorged adult male (n = 60) in New Hampshire is 0.03 g or 4–6% of that of an adult female (unpublished data, Pekins), and for easier comparison with previous studies, the analysis did not include this blood loss; however, the additive impact is addressed in the discussion.
Results
The following estimates were derived from calculations across a range of BM and condition (% BF) of adult moose cows, two forage consumption levels (1 and 1.2% BM), and a range of tick infestation (30,000–90000) and subsequent blood loss associated with the proportion of female ticks (25 and 50%). The principal physiological and bioenergetic data used to calculate energy balance and the energy impact from blood loss to winter ticks are provided in Tables 1, 2.

Table 1. Baseline energy estimates (kilojoules/day; kJ/d) used to calculate the winter energy balance of 3 size-classes of pregnant cow moose.
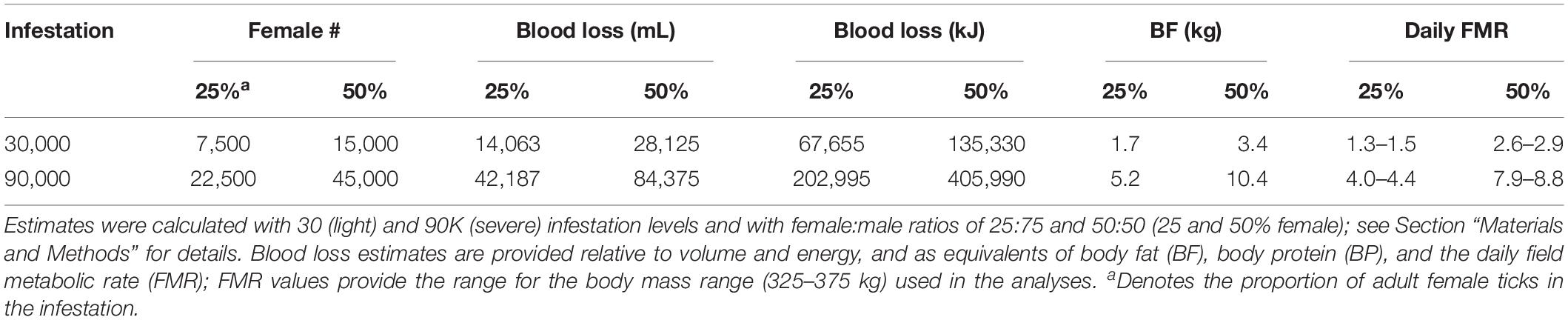
Table 2. Baseline estimates and values used to describe the energy impact from blood loss associated with feeding by adult female winter ticks on pregnant moose.
Cost of Gestation
To best evaluate the bioenergetic relationship between gestation and nutritional condition, it is informative to consider the cost of gestation alone (without winter ticks) relative to BM and% BF, as well as the cost for non-pregnant cows. The proportional cost of gestation was similar regardless of BM, on average ∼17% above FMR for the length of gestation. At low consumption, a non-pregnant small cow (325 kg, 15% BF) experiences a minor deficit in BF (1.5 kg) on 16 May (birth date if pregnant), and mid-sized and large cows retain 17.5 and 39 kg BF, respectively. Importantly, the maximum loss in BM is only 16% for the small cow at low consumption. At high consumption, a BF surplus occurs in all non-pregnant cows ranging from 19 (small cow) to 61 kg (large cow); albeit, the small cow has highest probability of not breeding.
For pregnant cows at birth (without infestation), a small cow experiences fat deficit at low (−28.4 kg) and high consumption (−8.8 kg), the mid-sized cow at low consumption (−9.4 kg), and the large cow has a fat surplus at low (11.8 kg) and high (34.4 kg) consumption. The estimated% loss in BM (combined fat and endogenous protein) at low and high consumption was 21–35, 18–26, and 16–22% for small, mid-sized, and large cows, respectively. Of consequence to small and mid-sized cows is that the proportional cost of lactation is 1.8× higher than the maximum cost of gestation at birth. Cows with a BF deficit at birth would lose ∼0.9 kg endogenous protein/d during the single week of lactation prior to green-up, elevating the overall loss in BM an additional 3–4%.
Cost of Gestation and Winter Ticks
As predicted, the energy balance equations demonstrated that starting BM or% BF was directly related to the nutritional condition at the end of gestation and through the first week of lactation. Only the large cow (375 kg, 25% BF) had any BF (1.4 kg) at birth in the worst-case scenario (high infestation and low consumption); at high consumption the cow maintained BF through the week of lactation regardless of consumption level (Figure 1). Conversely, in the best scenario (low infestation and high consumption) the small cow (325 kg, 15% BF) depleted BF ∼20 days before birth; in the worst scenario, the cow depleted BF ∼01 April or 45 days before birth. The mid-sized cow (350 kg, 20% BF) was intermediate of these extremes; in the best scenario it retained 6.5 kg BF at birth and in the worst scenario had a BF deficit of 19.8 kg (Figure 1).
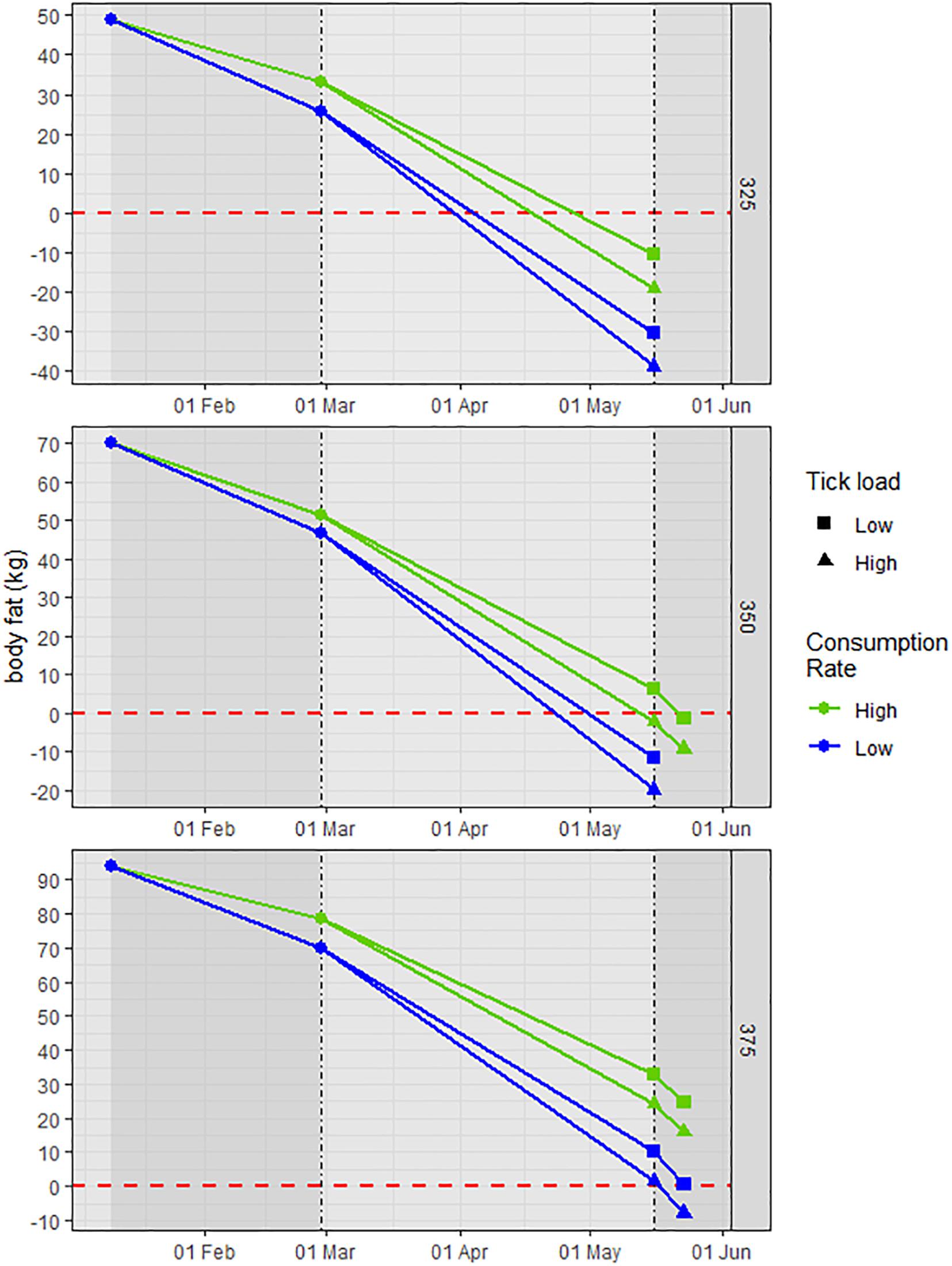
Figure 1. An illustration of the temporal loss of endogenous body fat (BF; kg) in adult cow moose of different starting body mass (325, 350, and 375 kg) and% BF (15, 20, and 25%) from 10 January through 16 May, a period encompassing part of the 2nd trimester and the 3rd trimester of gestation, and 1 week of lactation. The inflection points on 01 March and 16 May denote the 77-day period (01 March–16 May) of the 3rd trimester and 1 week of lactation (17–23 May). Consumption rate was varied as 1 and 1.2% body mass, and infestation was based on 90,000 ticks at two ratios of adult females – 25 and 50%. An energy balance equation was used to calculate the BF (kg) required to meet the energy deficit from the difference between the total daily energy cost (field metabolic rate, gestation, blood loss to ticks, and lactation) and consumption (kJ/d); the BF value on any given day represents the remaining endogenous BF (kg).
After fat depleted, I assumed that cows could no longer maintain BM and the decline in BM was equivalent to the estimated loss of endogenous protein (kg) to meet energy balance until birth. The caloric equivalent of fat is 2.3× that of protein (16.74 kJ/g) and this multiplier was used to convert the BF deficit (kg) on 16 May (birth) to an equivalent loss of endogenous protein/BM. The loss in BM associated with catabolism of endogenous protein was substantial in the small cow (range = 24–89 kg) and total loss in BM was 22–42% at birth across the two infestation levels. The mid-sized cow was in BF deficit only at low consumption when loss of endogenous protein ranged from 25.5 to 45.3 kg and total loss in BM was 27–33% BM at birth. The large cow did not experience BF deficit at any scenario until the first week of lactation at low consumption.
One unique approach of this exercise was to evaluate the effect of infestation level (30,000–90,000 ticks) and the ratio (25–50%) of adult female ticks on a moose at two consumption rates. At the highest infestation and proportion of adult females, the maximum loss in BF was ∼8 kg for any cow (Table 1), or a caloric equivalent of 6–7 days of FMR. As illustrated in Figure 2, the energetic cost of blood loss is concentrated for 8 weeks – Periods 2 (02–24 March) and 3 (25 March–22 April) – simultaneously and proportionally increasing along with gestation. The energy cost associated with blood loss in Period 3 elevates the total cost to the equivalent at maximal cost of gestation in Period 4 when blood loss is essentially zero. Extending this elevated cost (backward) from 4 to 8 weeks long accelerates the date of BF depletion and increases the days of rapid loss in BM associated with catabolism of endogenous protein, both of most consequence to the small cow. At low consumption, blood loss elevates total cost by 14% (small cow) to 12.5% (large cow) in Periods 2 and 3 (Figure 2), and slightly exceeds the cost of gestation in Period 2 for all cows.
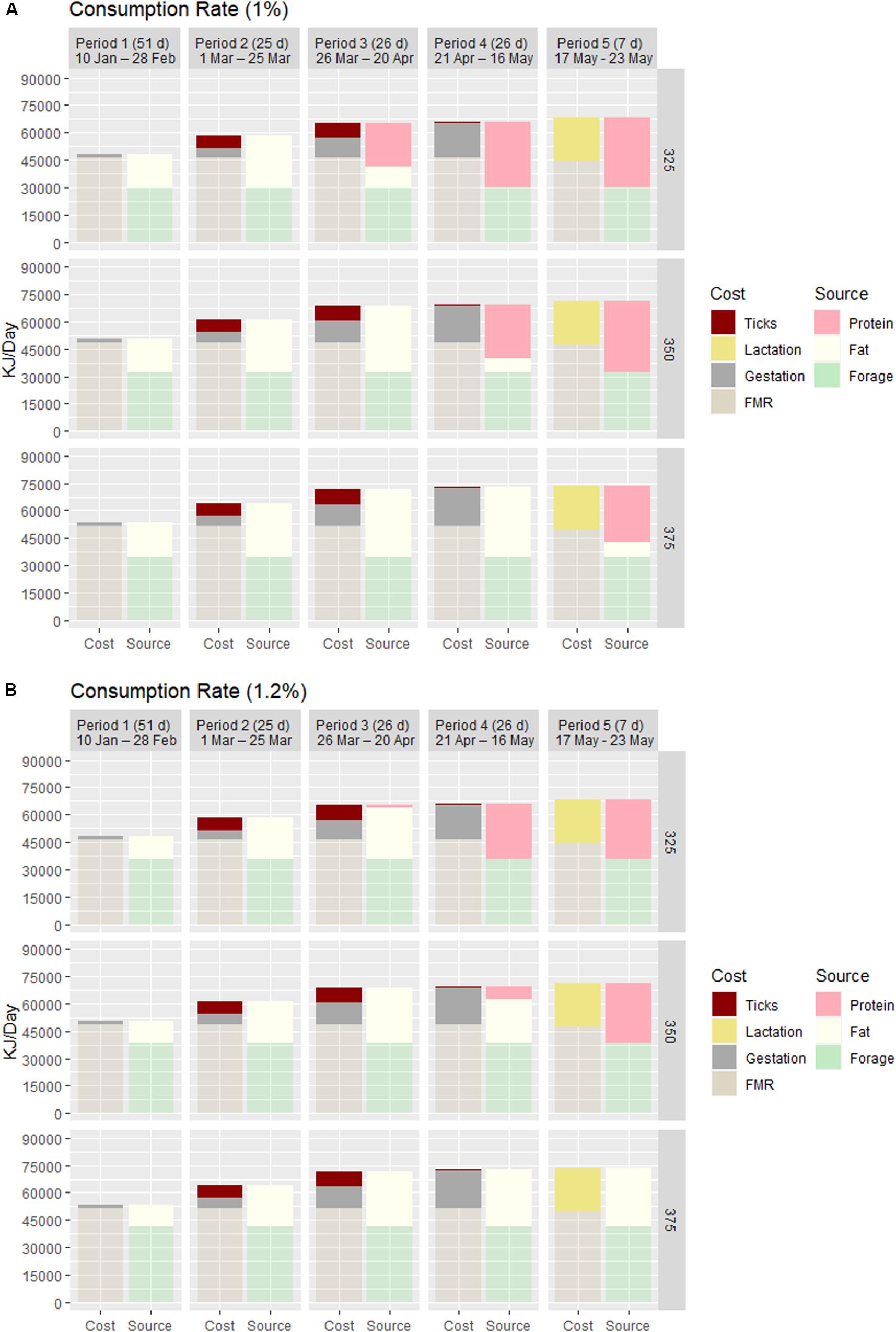
Figure 2. The proportional cost and source of the daily energy budget of adult cow moose of different starting body mass (325, 350, and 375 kg) and body fat (15, 20, and 25%) in five distinct periods from 10 January through 16 May. Period 1 was the latter part of the 2nd trimester of gestation (50 days), Periods 2–4 represented distinct periods (25, 26, and 26 days) of exponentially increasing cost of gestation with Periods 2 and 3 accounting for the concentrated blood loss to winter ticks, and Period 5 was lactation (7 days). The bars indicate how cost (field metabolic rate, gestation, blood loss, and lactation) was met relative to source (forage, BF, and BP) and the time frame at which a BF deficit occurs and BP is used. Consumption was varied as 1% (A) and 1.2% body mass (B) to illustrate its relative impact on energy balance.
Forage-consumption rates produced temporal differences in condition of all sized cows as illustrated in Figure 2. At high consumption, a measurable BF deficit occurs in Period 4 (post-ticks) for the small cow and during the 1-week lactation (Period 5) for mid-sized cow; the large cow does not experience a BF deficit through Period 5. In contrast, at low consumption all cows experienced measurable BF deficit one period earlier, with the small cow largely dependent on endogenous protein for most of the 3rd trimester of gestation. The small and mid-sized cows met the cost of lactation entirely with endogenous protein at both consumption rates.
Discussion
This exercise used a number of energetic estimates and assumptions that were simplified to more easily illustrate the relative influences of body mass, nutritional condition, and infestation level of winter ticks on pregnant cow moose; importantly, conservative estimates were used to benefit the nutritional condition of the cow. For example, FMR was set low relative to maintenance energy requirements, high estimates of forage value were applied throughout winter and “low consumption” was relative to that, only a single week of lactation was assumed prior to green-up, and catabolism of fat and muscle would not yield 100% of their energetic value as applied in the equations. Although blood-loss estimates assumed that the entire adult female infestation removed blood, this assumption ignored physical removal of ticks via shaking, grooming, and rubbing throughout the engorgement period (Addison et al., 2019). Conversely, negative behavioral, physical, and physiological responses to high tick loads including reduced forage consumption, increased grooming, and skin ailments were ignored (Samuel and Welch, 1991; Addison and McLaughlin, 1993; Mooring and Samuel, 1998; Addison et al., 2019), as was blood loss associated with all nymphs and adult male ticks. Nonetheless, because the infestation levels reflected the wide range of infestation measured on hides of dead calves through mid-April (∼20,000–95,000 ticks; Jones et al., 2019), the exercise arguably included a reasonable range of infestation and blood loss. All together, these analyses should be viewed in an evaluative context and conservative relative to the additive impact of winter tick infestation on individual productivity and population response of moose.
Paramount to the interpretation of such impact is that prior to spring green-up, moose have evolved to sustain adequate endogenous energy to complete gestation and 1–2 weeks of lactation. Pre-winter BM and condition are key to winter survival and productivity because moose cannot meet their energy or protein requirements from natural winter forage (Schwartz and Renecker, 2007; Parker et al., 2009), and like other northern ungulates, moose employ an overall energy conservation strategy to conserve endogenous resources. Declines in BM and condition are expected, and delayed fetal growth concentrating 90% of gestational cost in the 3rd and last trimester (Schwartz and Hundertmark, 1993; Schwartz, 2007) is considered an evolutionary strategy to confront the lack of digestible protein in winter browse (Robbins, 1993).
The difference in nutritional condition on 16 May (birth) between the small (325 kg) and large (375 kg) cows was evident, with or without tick infestation, and provides stark evidence of this evolutionary strategy (Figure 2). Even at high consumption rate and regardless of infestation level, the small cow depleted its BF by 22 April (end of Period 3, Figure 2; 3–4 weeks pre-birth), whereas, the large cow had surplus BF after the first week of lactation (Figures 1, 2). For the small cow, the rapid loss in BM associated with its catabolism of endogenous protein alone through late gestation and early lactation is likely not sustainable – total loss in BM would exceed 30%. The consequence is either mortal mass loss or failed pregnancy; given minimal adult mortality (Jones et al., 2019), the animal presumably copes through failed pregnancy (i.e., underdeveloped fetus and compromised neonate). For mid-sized cows, BM provides critical plasticity to meet the costs of late gestation and early lactation through the combined use of BF and BP without realizing mortal loss of BM (i.e., >30% BM). Although BF is paramount in the overall strategy, the importance of BP in late gestation and early lactation is clear and likely most important in longer winters when green-up is delayed (Parker et al., 2009).
It is not coincidental that pregnancy (∼80% annually; Jones et al., 2017) and birthing rates differ in the study population, and that abandoned, compromised, and still-born calves are located each year in the peak birthing period (Ellingwood, 2018). This difference is not uncommon in moose populations and reflects compromised body condition of pregnant cows as early as November–December (Testa and Adams, 1998) and/or late winter (Schwartz and Hundertmark, 1993). The low successive birthing rate in the population (Jones et al., 2019; Powers, 2019) indicates that a measurable proportion of cows are annually constrained by their pre-winter condition/BM. Although BM in autumn has been suggested as not predictive of productivity of moose in Norway (Milner et al., 2013), the pre-winter BM reflects relative condition, and this exercise illustrates that adequate BF is required to meet the combined cost of gestation and winter ticks in the critical 3rd trimester.
Unproductive cows are presumably compromised by inadequate compensatory growth in the previous summer-autumn from either the combined cost of gestation and infestation (325 kg cow) or raising a calf through summer (350 kg cow). Numerous studies have identified the relationship between condition and productivity in moose (e.g., Franzmann and Schwartz, 1985; Keech et al., 2000) and this exercise demonstrates the necessity of adequate BF to successfully reproduce. Of most consequence is that the cost of gestation is focused nearly entirely (90%) in the last trimester at end of winter, that the entirety of gestation and early lactation occur prior to spring green-up, and that decline in BM is rapid (2.3×) after depletion of BF. The near complete failure of surviving female calves to ovulate/breed as yearlings similarly reflects inadequate compensatory growth in their second summer to overcome low post-winter BM; the threshold yearling BM (field-dressed) associated with ovulation is 200 kg (Adams and Pekins, 1995).
Variability in successive calving rate was high in the current study (2015–2019). Equal proportions of adult cows calved 2 years in succession, and every other year; few calved 3–5 years continuously, and some were unsuccessful for 2 consecutive years—calving success was defined as multiple observations (imperfect) of a live calf in summer (Powers, 2019). Cows failing to produce a viable or surviving calf avoid the cost of lactation and benefit most from compensatory growth during summer-autumn to produce the following year (Parker et al., 2009; Shively et al., 2019); further, non-pregnant cows avoid the cost of both gestation and lactation, although the annual pregnancy rate was ∼80% (Powers, 2019). Examples of constraining winters causing lag in population recovery of ungulates in North America include white-tailed deer (Patterson and Power, 2002; Garroway and Broders, 2007), moose (Heard et al., 1997; Testa and Adams, 1998), and caribou (Rangifer tarandus; Cameron, 1994; Allaye Chan-McLeod et al., 1999). Arguably, winter ticks act in an analogous manner with moose in the Northeast via direct mortality and reduced productivity, except epizootics can occur regardless of winter conditions (Musante et al., 2010; Jones et al., 2019). Although the “selfish cow” theory suggests that an adult cow might pause pregnancy for self-maintenance in severe environmental conditions (Russell et al., 1998, 2005; Morano et al., 2013) and delaying reproduction could lead to higher lifetime production (Festa-Bianchet and Cote, 2008 in Parker et al., 2009), this advantage is unlikely during continuous and long-term parasitism by winter ticks.
The difference between the pregnancy and calving rates, and that dead and under-developed neonates are located in the field (Ellingwood, 2018), points to an imbalance between endogenous resources and the cost of gestation. Rapid loss in BM associated with catabolism of BP should not occur prior to the 3rd trimester (01 March) unless BF is depleted; in no scenario did that occur, and even the small cow retained BF into early April at low consumption and high infestation (Period 3, Figure 2). Therefore, rapid loss in BM prior to the 3rd trimester would require extremely poor condition (i.e., without BF) in mid-winter. Although perinatal mortality on poor winter habitat was proposed in a Norwegian study (Milner et al., 2013), the probability of such would be rare based on this analysis, and it is counter to the evolutionary strategy to delay the cost of gestation until the 3rd trimester. The analyses point to all cows reaching the 3rd trimester with some BF, and that successful calving will be most influenced by subsequent balance of endogenous fat and protein against the exponentially increasing cost of gestation and the concentrated blood loss to winter ticks. All cows could sustain these costs for multiple days with BP alone, and it is more likely that failed calving involves an underdeveloped fetus, compromised calf, or predisposed predation versus absorption.
Although this analysis identifies adequate BF as a prerequisite for successful reproduction, BP is undeniably critical in meeting the cost of fetal growth, particularly during the 3rd trimester when >80% of growth occurs. The 15 kg newborn/18 kg conceptus mass (15–18% protein in related species; Robbins and Moen, 1975; Oftedal, 1985) is roughly equivalent to the loss (use) in lean BM (25% protein); importantly, this loss occurs simultaneously and prior to depletion of BF. Blood loss associated with winter ticks in late March–April represents an additional loss of BP during the 3rd trimester. At moderate-high infestation (30,000–70,000 ticks), blood loss was estimated as 28–42% of the daily maintenance protein requirement of a 360 kg non-pregnant cow, a proportional impact exceeding that associated with the daily energy requirement (Musante et al., 2007). Relative to that maintenance protein requirement (Schwartz et al., 1987; Schwartz and Renecker, 2007), blood loss at infestations of 30,000 and 90,000 ticks requires 2.8–16.9 kg protein (Table 2), or an equivalent loss of 11–68 kg of lean BM; ∼3 to 20% of BM from lowest to highest infestation. Similarly, DelGiudice et al. (1997) estimated daily loss of 0.5–0.8 kg lean BM/d in a 400 kg moose at infestation of ∼30,000 ticks in a nutritionally restricted population on Isle Royale. Extrapolation across 8 weeks of feeding by adult female ticks equals ∼36 kg of lean BM, assuming a constant rate of loss. Critical to moose is that the winter diet is protein-deficient relative to the daily requirement, hence, winter ticks accelerate the use of BP and BM loss to address the additive cost of blood loss. Importantly, this specific loss in lean BM was not captured in the energy balance equation. But it is substantial and mostly disadvantages small and mid-sized cows by further reducing the probability of successful calving. Not coincidentally, mortality of 10-month old calves is ultimately a consequence of acute anemia as BP and lean BM deplete in face of concentrated blood loss that can exceed the total blood volume in 3–4 weeks at infestations of 30,000+ ticks (Musante et al., 2007; Jones et al., 2019).
With regard to compensatory growth in summer, the nutritional importance and availability of summer forage, specifically dietary protein, is paramount to the autumnal condition (BM and BF) of cows. Surprisingly, forage intake rates of lactating and non-lactating cows in Alaska were similar to each other and to predicted maximum rates, or that all animals maximized intake in summer (Shively et al., 2019). Thus, lactating cows were necessarily smaller, averaging 32 kg less than their unproductive counterparts in autumn; presumably, this difference would be largely reflected in lower BF. The impact of winter ticks (relative to the infestation level) would be to lower nutritional condition in both these groups entering summer, ultimately reducing pre-winter BM and BF of all animals with highest penalty for lactating cows. Arguably, the study population reflects the annual interactions of multiple factors in the face of continually high, annual infestation of winter ticks —body condition and energy balance, protein balance and requirements as influenced by late-term gestation and blood loss to winter ticks, and differential compensatory growth relative to the cost of lactation and setting pre-winter condition —with productivity the annual population response.
Low reproductive rate and BM is associated with resource constraints in a traditional assessment of nutritional carrying capacity; however, resource and habitat constraints are presumed minimal for moose in the Northeast that is characterized as excellent moose habitat (Scarpitti et al., 2005; Dunfey-Ball, 2017). Typically, these physiological outcomes are relieved either through improved environmental conditions (e.g., habitat quality and winter conditions) or lower population density (direct or indirect), with only the latter of near-term consequence in the Northeast. A density-dependent relationship presumably exists between moose and winter ticks relative to annual infestation and moose mortality, and Samuel (2007) posits densities of <1 and >∼3 moose/km2 in Ontario as respective thresholds for minimal and epizootic-level impacts by winter ticks. In comparison, during the recent period of frequent epizootics since the mid-2000s, the density estimate in New Hampshire has declined from 0.87 to 0.46 moose/km2. Assuming these density estimates are relatively accurate, this lower density is sustaining sufficient abundance of winter ticks to cause frequent epizootics and reduced productivity in the study area. Given that density estimates are typically regional in nature, it is important to recognize that density and impacts could be higher at the local scale, and that patterns in forest harvesting and foraging behavior are related directly to and influence moose and tick abundance (Healy et al., 2018, 2020; Powers and Pekins, 2020). In common throughout the Northeast are broad-scale commercial forest harvesting that promotes optimal moose habitat and warming temperatures that increase the autumnal questing period of winter ticks and subsequent infestation of moose. Further study is warranted to better understand and predict the influence of these interrelationships that are principal factors in the population dynamics of moose in the Northeast.
In more northern regions, substantial increases in moose populations are typically associated with major environmental perturbations (e.g., fire) that result in long-term population cycles (see Peek, 2007); further, predation often plays an important role in the dynamics of northern populations (Ballard and Van Ballenberghe, 2007). Similarly, the rapid moose expansion that occurred in the Northeast originated from extensive harvest of a large swathe of contiguous forestland across three states in only 15–20 years (Chen et al., 2017). The temporal difference in these two situations is that commercial forest harvesting continues to maintain high availability of optimal foraging habitat in the Northeast (15–20% of forestland; Dunfey-Ball, 2017). Given the density-dependent relationship between moose and winter ticks, it is intriguing to consider whether predation might act as an ameliorating factor against build-up of winter ticks given epizootics were relatively uncommon historically, and whether winter ticks slowly act as a “de facto” predator in the Northeast; albeit, all animals are potentially “harmed” by winter ticks.
It follows that winter tick density grew with increasing moose density, and by the mid-2000s the first epizootic was identified (Musante et al., 2010), with epizootics suspected or directly measured with unprecedented frequency since (Ellingwood et al., 2020). Surprisingly, a single year of “low” calf mortality (still 30%) occurred in 2017 when infestation was the lowest measured in the study, presumably due to the effect of September drought on larval survival and abundance (Dunfey-Ball, 2017; Ellingwood et al., 2019). This reprieve, however, was short-lived as the autumnal abundance of winter ticks was enough to induce an epizootic in spring 2018 (Ellingwood et al., 2020). Again, the unprecedented frequency and persistence of these population impacts illustrate that a unique moose-winter tick relationship operates in the Northeast and that shorter winters and warming temperatures in autumn and spring are directly beneficial to winter ticks and subsequently detrimental to moose.
I note that both the rapid geographic expansion and growth, and current slow decline of this very large regional population (>50,000 animals) occurred since the 1980s. This time frame provides little support for the idea of thermal stress in moose operating at the individual or population level or in the Northeast; how could growth in this regional population occur while others decline in similar environmental conditions? As raised by Montgomery et al. (2019), this relationship is invariably correlative when presented (e.g., Lenarz et al., 2009) and simplified by comparing ambient temperature to the upper critical temperature (UCT) reported by Renecker and Hudson (1986); obvious physical and productivity data (e.g., BM and twinning rate) that contradict such a conclusion are oftentimes ignored. Further, thermoregulatory data or predictions based on air temperature alone are not applicable to a free-ranging animal, and with regard to the most regularly cited study (Renecker and Hudson, 1986), data are both minimal (two animals) and highly variable in summer; those researchers would agree that caution should be used in any extrapolation. Although individual animals obviously experience thermal stress and adjust to it behaviorally (e.g., Lowe et al., 2010; Montgomery et al., 2019), it should be noted that extrapolation of the summer UCT (Renecker and Hudson, 1986) places nearly all moose under constant “thermal stress” throughout the productive summer season, a physiological impossibility and primary evidence to refrain from such simplistic correlations.
Climate change affects winter length at both ends (Williams et al., 2015), and length (days) of severe winter conditions, not the conditions per se, best predicts weather-associated population impacts; for example, winter severity indices for white-tailed deer typically total the number of “severe days” (Nelson, 1995). Because warming temperatures should induce earlier spring green-up, there is irony relative to the negative influence caused by extended questing of winter ticks in autumn versus the positive influence realized from an improved spring diet during late gestation and early lactation. It is important to recognize that ungulates occupying seasonal environments may be positively (Tape et al., 2016) or negatively (Monteith et al., 2015) affected by measurable change in vegetation/habitat, and might also benefit, or not, from subtle changes within an annual seasonal cycle. For example, a 2-week earlier green-up would measurably reduce endogenous tissue loss, provide a higher quality diet prior to conception, and aid in early compensatory growth. Conversely, a 2-week extension of the autumnal questing season of winter ticks represents a potential 20+% increase of infestation assuming a questing period of 9 weeks (September through early November). No empirical data exist to evaluate the relative influence of winter tick parasitism versus diet improvement from an earlier spring, but an earlier spring would also provide advantageous ground conditions for maximum survival of adult females and larvae (Drew and Samuel, 1986; Addison et al., 1998; Yoder et al., 2016; Holmes et al., 2018). Considering that the physiological impact of winter ticks is directly related to infestation level that affects survival of 10-month old calves, subsequent productivity in surviving yearlings (Musante et al., 2007; Jones et al., 2019), condition during and after gestation and lactation, and that epizootics occur regardless of winter conditions in the Northeast (Musante et al., 2010; Jones et al., 2019), a warming climate should be considered a constant negative to moose in the Northeast at this time.
The moose-winter tick relationship provides a prime example of how the influence of warming temperatures occurs most commonly and directly at the insect, parasite, and disease levels that are directly impacted by minimal change in ambient temperature (Leighton et al., 2012), and indirectly on medium-sized and large mammals like moose that regularly practice thermoregulatory behavior (e.g., Wattles et al., 2018; Montgomery et al., 2019). Previous research has identified the negative impact of sustained high abundance of winter ticks on survival and productivity of moose in the Northeast, and this analysis identifies the relationship between declining condition of adult cows and productivity (Keech et al., 2000) as influenced by the correlation between BM and BF (Sand et al., 1995). Further, the low successive birthing rate reflects that individual condition and annual productivity are influenced by reproductive success the previous year in this system.
Exposed to frequent high infestation of winter ticks, it follows that this population has higher than normal proportions of small and mid-sized cows most vulnerable to depletion of BF during gestation and lactation, and ultimately, unsuccessful calving. The long-term trend is decline in field-dressed weights and corpora lutea counts in yearling and adult cows from 1999–2009 to 2005–2009 (Bergeron et al., 2013). Because yearlings are unproductive in the study area (Jones et al., 2017, 2019), surviving female calves are compromised relative to their potential lifetime productivity (Gaillard et al., 2003). Use of demographic data from the mid-2000s and 2014–2019 in population models (Ellingwood et al., 2020) reveals the stark impact on population trajectory at the current high frequency of epizootics (high infestation), with potential halving of the population in as few as 10 years. Although this conservative analysis likely underestimates individual impact, because adequate if not optimal habitat is available, the moose population should respond rapidly upon release from high infestation. Interestingly, the single year (2017) of low infestation had 15% higher calving success than the 6-year average (2014–2019; Powers, 2019).
Relative to weather and climate, further study is warranted to better interpret the dynamic interactions and relationships between and among moose and tick densities, habitat use and infestation of moose, and ground conditions and winter tick abundance. Use of empirical data from the Northeast in a predictive, agent-based model has yielded insights about a self-sustaining, habitat use-infestation relationship (Healy et al., 2020), and subsequent field estimates of larval tick abundance (Powers and Pekins, 2020) provide supporting evidence at the micro-site level. Further, population modeling indicates that population stability in the Northeast occurs at an epizootic frequency of 1 in 4 years (Ellingwood et al., 2020), yet the current rate is 5 in 6 and 7 in the past 10 years. Clearly, moose are confronted with an historically unique combination of environmental and parasitic conditions associated with a warming climate that markedly affects their survival and productivity; in effect, winter tick parasitism represents the major constraint in the nutritional carrying capacity of moose in the Northeast.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
Funding for this project was provided in part through Wildlife Restoration Program grant No. F13AF01123 (NH W-104-R-1) to New Hampshire Fish and Game Department from the United States Fish and Wildlife Service, Division of Wildlife and Sport Fish Restoration with matching funds provided by the University of New Hampshire.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many “moose” grads collected the bulk of the empirical data used in the analysis – A. Musante, D. Scarpitti, M. Eckert, D. Bergeron, H. Andreozzi, H. Jones, N. Fortin, K. Dunfey-Ball, D. Ellingwood, C. Healy, and B. Powers supervised tens of undergraduate students including the most reliable J. DeBow and T. Soucy. A. Siren constructed the figures and provided endless editing, improvements, and ideas. I am thankful to work with such motivated and talented students.
References
Adams, K. P., and Pekins, P. J. (1995). Growth patterns of New England moose: yearlings as indicators of population status. Alces 31, 53–59.
Addison, E. M., Fraser, D. J. H., and McLaughlin, R. F. (2019). Grooming and rubbing behavior by moose experimentally infested with winter ticks (Dermacentor albipictus). Alces 55, 23–35.
Addison, E. M., Joachim, D. G., McLaughlin, R. F., and Fraser, D. J. H. (1998). Ovipositional development and fecundity of Dermacentor albipictus (Acari: Ixodidae) from moose. Alces 34, 165–172.
Addison, E. M., and McLaughlin, R. F. (1993). Seasonal variation and effects of winter ticks (Dermacentor albipictus) on consumption of food by captive moose (Alces alces) calves. Alces 29, 219–224.
Allaye Chan-McLeod, A. C., White, R. G., and Russell, D. E. (1999). Comparative body composition strategies of breeding and nonbreeding female caribou. Can. J. Zool. 77, 1901–1907. doi: 10.1139/z99-169
Andreozzi, H. A., Pekins, P. J., and Langlais, M. L. (2014). Impact of moose browsing on forest regeneration in Northeast vermont. Alces 50, 67–79.
Ballard, W. B., and Van Ballenberghe, V. (2007). “Predator/Prey relationships,” in Ecology and Management of the North American Moose, eds A. W. Franzmann and C. C. Schwartz (Boulder, CO: University Press of Colorado), 247–273.
Bergeron, D. H., Pekins, P. J., Jones, H. F., and Leak, W. B. (2011). Moose browsing and forest regeneration: a case study. Alces 47, 39–51.
Bergeron, D. H., Pekins, P. J., and Rines, K. (2013). Temporal assessment of physical characteristics and reproductive status of moose in New Hampshire. Alces 49, 39–48.
Bontaites, K. M., and Gustafson, K. (1993). The history and status of moose management in New Hampshire. Alces 29, 163–167.
Cameron, R. D. (1994). Reproductive pauses by female caribou. J. Mammal. 75, 10–13. doi: 10.2307/1382230
Chen, C., Weiskittel, A., Bataineh, M., and MacLean, D. A. (2017). Evaluating the influence of varying levels of spruce budworm defoliation on annualized individual tree growth and mortality in Maine, USA and New Brunswick, Canada. For. Ecol. Manage. 396, 184–194. doi: 10.1016/j.foreco.2017.03.026
DelGiudice, G. D., Peterson, R. O., and Samuel, W. M. (1997). Trends of winter nutritional restriction, ticks, and numbers of moose in Isle Royale. J. Wildl. Manage. 61, 895–903.
Drew, M. L., and Samuel, W. M. (1986). Reproduction of the winter tick, Dermacentor albipictus, under field conditions in Alberta, Canada. Can. J. Zool. 64, 714–721. doi: 10.1139/z86-105
Drew, M. L., and Samuel, W. M. (1989). Instar development and disengagement rate of engorged female winter ticks, Dermacentor albipictus (Acari: Ixodidae), following single- and trickle-exposure of moose (Alces alces). Exp. Appl. Acarol. 6, 189–196. doi: 10.1007/BF01193979
Dunfey-Ball, K. R. (2017). Moose Density, Habitat, and Winter Tick Epizootics in a Changing Climate. Master’s Thesis, University of New Hampshire, Durham, NH.
Eckert, M. D. (2004). Estimating Field Metabolic Rate of Captive Deer in Winter: A Comparison of Three Methods. Master’s Thesis, University of New Hampshire, Durham, NH.
Ellingwood, D. (2018). Assessing the Impact of Winter Tick Epizootics on Moose Condition and Population Dynamics in Northern New Hampshire. Master’s Thesis, University of New Hampshire, Durham, NH.
Ellingwood, D., Pekins, P. J., and Jones, H. (2019). Using snow urine samples to assess the impact of winter ticks on moose calf condition and survival. Alces 55, 13–21.
Ellingwood, D., Pekins, P. J., Jones, H., and Musante, A. R. (2020). Evaluating moose (Alces alces) population response to infestation level of winter ticks (Dermacentor albipictus). Wildl. Biol. doi: 10.2981/wlb.00619
Festa-Bianchet, M., and Cote, S. D. (2008). Mountain Goats: Ecology, Behavior, and Conservation of an Alpine Ungulate. Washington, D.C: Island Press.
Franzmann, A. W., and Schwartz, C. C. (1985). Moose twinning rates: a possible population condition assessment. J. Wildl. Manage. 49, 394–396. doi: 10.2307/3801540
Gaillard, J.-M., Loison, A., Toigo, C., Delmore, D., and Van Laere, G. (2003). Cohort effects and deer population dynamics. Ecoscience 10, 412–420. doi: 10.1080/11956860.2003.11682789
Garroway, C. J., and Broders, H. G. (2007). Adjustment of reproductive investment and offspring sex ratio in whiote-taileed deer (Odocoileus virginianus) in Nova Scotia. Can. J. Mammal. 88, 1305–1311. doi: 10.1644/06-mamm-a-212r2.1
Glines, M. V. (1983). The Winter Tick, Dermacentor Albipictus (Packard, 1869): Its Life History, Development at Constant Temperatures and Physiological Effects On Moose, Alces Alces L. Master’s thesis, University of Alberta, Edmonton.
Healy, C., Pekins, P. J., Attallah, S., and Congalton, R. G. (2020). Using agent-based models to inform the dynamics of winter tick parasitism of moose. Ecol. Complex 41:100813. doi: 10.1016/j.ecocom.2020.100813
Healy, C., Pekins, P. J., Kantar, L., Congalton, R. G., and Atallah, S. (2018). Selective habitat use by moose during critical periods in the winter tick life cycle. Alces 54, 85–100.
Heard, D., Barry, S., Watts, G., and Child, K. (1997). Fertility of female moose (Alces alces) in relation to age and body condition. Alces 33, 165–176.
Holmes, C. J., Dobrotka, C. J., Farrow, D. W., Rosendale, A. J., Benoit, J. B., Pekins, P. J., et al. (2018). Low and high thermal tolerance characteristics for unfed larvae of the winter tick Dermacentor albipictus (Acari: Ixodidae) with special reference to moose. Ticks Tick. Borne. Dis. 9, 25–30. doi: 10.1016/j.ttbdis.2017.10.013
Jones, H., Pekins, P., Kantar, L., Sidor, I., Ellingwood, D., Lichtenwalner, A., et al. (2019). Mortality assessment of moose (Alces alces) calves during successive years of winter tick (Dermacentor albipictus) epizootics in New Hampshire and Maine (USA). Can. J. Zool. 97, 22–30. doi: 10.1139/cjz-2018-0140
Jones, H., Pekins, P. J., Kantar, L. E., and Ellingwood, D. (2017). Fecundity and summer calf survival of moose during 3 successive years of winter tick epizootics. Alces 53, 85–98.
Keech, M. A., Bowyer, R. T., Hoef, J. M., Ver Boertje, R. D., Dale, B. W., and Stephenson, T. R. (2000). Life-history consequences of maternal condition in alaskan moose. J. Wildl. Manage. 64, 450–462. doi: 10.2307/3803243
Leighton, P. A., Koffi, J. K., Pelcat, Y., Lindsay, L. R., and Ogden, N. H. (2012). Predicting the speed of tick invasion: an empirical model of range expansion for the lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 49, 457–464. doi: 10.1111/j.1365-2664.2012.02112.x
Lenarz, M. S., Nelson, M. E., Schrage, M. W., and Edwards, A. J. (2009). Temperature mediated moose survival in northeastern Minnesota. J. Wildl. Manage. 73, 503–510. doi: 10.2193/2008-265
Lowe, S. J., Patterson, B. R., and Schaefer, J. A. (2010). Lack of behavioral responses of moose (Alces alces) to high ambient temperatures near the southern periphery of their range. Can. J. Zool. 88, 1032–1041. doi: 10.1139/Z10-071
Milner, J. M., van Beest, F. M., Solberg, E. J., and Storaas, T. (2013). Reproductive success and failure: the role of winter body mass in reproductive allocation in Norwegian moose. Oecologia 172, 995–1005. doi: 10.1007/s00442-01202547-x
Monteith, K. L., Klaver, R. W., Hersey, K. R., Holland, A. A., Thomas, T. P., and Kauffman, M. J. (2015). Effects of climate and plant phenology on recruitment of moose at the southern extent of their range. Oecologia 178, 1137–1148. doi: 10.1007/s00442-015-3296-4
Montgomery, R. A., Redilla, K. M., Moll, R. J., Van Moorter, B., Rolandsen, C. M., Millspaugh, J. J., et al. (2019). Movement modeling reveals the complex nature of the response of moose to ambient temperatures during summer. J. Mammal. 100, 169–177. doi: 10.1093/jmammal/gyy185
Mooring, M. S., and Samuel, W. M. (1998). The biological basis of grooming in moose: programmed versus stimulus-driven grooming. Anim.Behav. 56, 1561–1570. doi: 10.1006/anbe.1998.0915
Morano, S., Stewart, K. M., Sedinger, J. S., Nicolai, C. A., and Vavra, M. (2013). Life-history strategies of North American elk: trade-offs associated with reproduction and survival. J. Mammal. 94, 162–172. doi: 10.1644/12-mamm-a-074.1
Musante, A., Pekins, P., and Scarpitti, D. (2007). Metabolic impacts of winter tick infestations on calf moose. Alces 43, 101–110.
Musante, A. R., Pekins, P. J., and Scarpitti, D. L. (2010). Characteristics and dynamics of a regional moose Alces alces population in the northeastern United States. Wildl. Biol. 16, 185–204. doi: 10.2981/09-014
Nelson, M. E. (1995). Winter range arrival and departure of white-tailed deer in northeastern Minnesota. Can. J. Zool. 73, 1069–1076. doi: 10.1139/z95-127
Oftedal, O. T. (1985). “Pregnancy and lactation,” in Bioenergetics of Wild Herbivores, eds R. J. Hudson and R. G. White (Boca Raton, FL: CRC Press), 216–238.
Parker, K. L., Barboza, P. S., and Gillingham, M. P. (2009). Nutrition integrates environmental responses of ungulates. Funct. Ecol. 23, 57–69. doi: 10.1111/j.1365-2435.2009.01528.x
Parker, K. L., Gillingham, M. P., Hanley, T. A., and Robbins, C. T. (1999). Energy and protein balance of free-ranging black-tailed deer in a natural forest environment. Wildl. Monogr. 143, 1–48.
Patterson, B. R., and Power, V. A. (2002). Contributions of forage competition, harvest, and climate fluctuation to changes in population growth of northern white-tailed deer. Oecologia 13, 62–71. doi: 10.1007/s004420100783
Peek, J. M. (2007). “Habitat relationships,” in Ecology and Management of the North American Moose, eds A. W. Franzmann and C. C. Schwartz (Boulder, CO: University Press of Colorado), 351–375.
Pekins, P. J. (1995). Winter Energy and Activity Budgets of Free-Ranging Deer in Northern New Hampshire. Final Report. Pittman-Robertson Grant W-12-R. Concord, NH: New Hampshire Fish and Game Department.
Pekins, P. J., Smith, K. S., and Mautz, W. W. (1998). The energy cost of gestation in white-tailed deer. Can. J. Zool. 76, 1091–1097. doi: 10.1139/z98-032
Powers, B. L. (2019). Assessing the Relationships of Winter Ticks, Weather and A Declining Moose Population in Northern New Hampshire. Master’s Thesis, University of New Hampshire, Durham.
Powers, B. L., and Pekins, P. J. (2020). Abundance of winter ticks (Dermacentor albipictus) in two regenerating forest habitats in New Hampshire, USA. Alces 56, 1–13.
Reese, E. O., and Robbins, C. T. (1994). Characteristics of moose lactation and neonatal growth. Can. J. Zool. 72, 953–957. doi: 10.1139/z94-130
Regelin, W. L., Schwartz, C. C., and Franzmann, A. W. (1985). Seasonal energy metabolism of adult moose. J. Wildl. Manage. 49, 394–396. doi: 10.2307/3801539
Renecker, L. A., and Hudson, R. J. (1986). Seasonal energy expenditures and thermoregulatory responses of moose. Can. J. Zool. 64, 322–327. doi: 10.1139/z86-052
Robbins, C. T., and Moen, A. N. (1975). Uterine compositin and growth in pregnant white-tailed deer. J. Wildl. Manage. 39, 684–691.
Russell, D. E., Gerhart, K. L., White, R. G., and Van De Wetering, D. (1998). Detection of early pregnancy in caribou: evidence for embryonic mortality. J. Wildl. Manage. 62, 1966–1975. doi: 10.2307/3802559
Russell, D. E., White, R. G., and Daniel, C. J. (2005). Energetics of the Porcupine Caribou Herd: A Computer Simulation Model. Ottawa: Canadian Wildlife Service.
Samuel, W. M. (2004). White as A Ghost: Winter Ticks and Moose. Natural History Series. Edmonton: Federation of Alberta Naturalists.
Samuel, W. M. (2007). Factors affecting epizootics of winter ticks and mortality of moose. Alces 43, 39–48.
Samuel, W. M., and Welch, D. A. (1991). Winter ticks on moose and other ungulates: factors influencing their population size. Alces 27, 169–182.
Sand, H., Cederlund, G., and Danell, K. (1995). Geographical and latitudinal variation in growth patterns and adult body size of Swedish moose (Alces alces). Oecologia 102, 433–442. doi: 10.1007/BF00341355
Scarpitti, D., Habeck, C., Musante, A. R., and Pekins, P. J. (2005). Integrating habitat use and population dynamics of moose in northern New Hampshire. Alces 41, 25–35.
Schwartz, C. C. (2007). “Reproduction, natality and growth,” in Ecology and Management of the North American Moose, eds A. W. Franzmann and C. C. Schwartz (Boulder, CO: University Press of Colorado), 141–171.
Schwartz, C. C., Hubbert, M. E., and Franzmann, A. W. (1988a). Changes in body composition of moose during winter. Energy requirements of adult moose for winter maintenance. Alces 24, 178–187.
Schwartz, C. C., Hubbert, M. E., and Franzmann, A. W. (1988b). Energy requirements of adult moose for winter maintenance. J. Wildl. Manage. 52, 26–33. doi: 10.2307/3801052
Schwartz, C. C., and Hundertmark, K. J. (1993). Reproductive characteristics of Alaskan moose. J. Wildl. Manage. 57, 454–468. doi: 10.2307/3809270
Schwartz, C. C., Regelin, W. L., and Franzmann, A. W. (1987). Protein digestion in moose. J. Wildl. Manage. 55, 352–357.
Schwartz, C. C., and Renecker, L. A. (2007). “Nutrition and energetics,” in Ecology and Management of the North American Moose, eds A. W. Franzmann and C. C. Schwartz (Boulder, CO: University Press of Colorado), 441–478.
Shively, R. D., Crouse, J. A., Thompson, D. P., and Barboza, P. S. (2019). Is summer food intake a limiting factor for boreal browsers? Diet, temperature, and reproduction as drivers of consumption in female moose. PLoS One 14:e0223617. doi: 10.1371/journal.pone.0223617
Tape, K. D., Gustine, D. D., Ruess, R. W., Adams, L. G., and Clark, J. A. (2016). Range expansion of moose in arctic Alaska linked to warming and increased shrub habitat. PLoS One 11:e0152636. doi: 10.1371/journal.pone.0152636
Tarr, M. D., and Pekins, P. J. (2002). Influences of winter supplemental feeding on the energy balance of white-tailed deer fawns in New Hampshire, U.S.A. Can. J. Zool. 80, 6–15. doi: 10.1139/z01-200
Testa, J. W., and Adams, G. P. (1998). Body condition and adjustments to reproductive effort in female moose (Alces alces). J. Mammal. 79, 1345–1354. doi: 10.2307/1383026
Wattles, D. W., Zeller, K. A., and DeStefano, S. (2018). Range expansion in unfavorable environments through behavioral responses to microclimatic conditions: moose (Alces americanus) as the model. Mamm. Biol. 93, 189–197. doi: 10.1016/j.mambio.2018.05.009
Williams, C. M., Henry, H. A. L., and Sinclair, B. J. (2015). Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol. Rev. 90, 214–235. doi: 10.1111/brv.12105
Keywords: body fat, climate change, energy balance, gestation, moose, body protein, productivity, winter ticks
Citation: Pekins PJ (2020) Metabolic and Population Effects of Winter Tick Infestations on Moose: Unique Evolutionary Circumstances? Front. Ecol. Evol. 8:176. doi: 10.3389/fevo.2020.00176
Received: 31 January 2020; Accepted: 19 May 2020;
Published: 16 June 2020.
Edited by:
R. Terry Bowyer, University of Alaska Fairbanks, United StatesReviewed by:
Brent Patterson, Ontario Ministry of Natural Resources and Forestry, CanadaPerry S. Barboza, Texas A&M University, United States
Copyright © 2020 Pekins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Pekins, pete.pekins@unh.edu
 Peter J. Pekins
Peter J. Pekins