Abstract
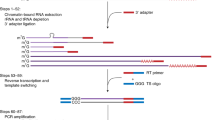
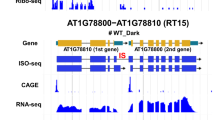
In eukaryotes, genes are transcribed by RNA polymerase-II (Pol-II) and introns are removed by the spliceosome largely cotranscriptionally1,2,3; analysis using long-read sequencing revealed that splicing occurs immediately after Pol-II passes introns in yeast4,5. Here, we developed a Nanopore-based method to profile chromatin-bound RNA that enables the simultaneous detection of splicing status, Pol-II position and polyadenylation at the genome-wide scale in Arabidopsis. We found that more than half of the introns remain unspliced after Pol-II transcribes 1 kb past the 3′ splice site, which is much slower than the rate of splicing reported in yeast4,5. Many of the full-length chromatin-bound RNA molecules are polyadenylated, yet still contain unspliced introns at specific positions. These introns are nearly absent in the cytoplasm and are resistant to nonsense-mediated decay, suggesting that they are post-transcriptionally spliced before the transcripts are released into the cytoplasm; we therefore termed these introns post-transcriptionally spliced introns (pts introns). Analysis of around 6,500 public RNA-sequencing libraries found that the splicing of pts introns requires the function of splicing-related proteins such as PRMT5 and SKIP, and is also influenced by various environmental signals. The majority of the intron retention events in Arabidopsis are at pts introns, suggesting that chromatin-tethered post-transcriptional splicing is a major contributor to the widespread intron retention that is observed in plants, and could be a mechanism to produce fully spliced functional mRNAs for rapid response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated in this study were deposited at NCBI under the accession number PRJNA591665.
Code availability
The code used to perform Poly(A) tail analysis is available at https://github.com/zhailab/polyACaller.
References
Merkhofer, E. C., Hu, P. & Johnson, T. L. Introduction to cotranscriptional RNA splicing. Methods Mol. Biol. 1126, 83–96 (2014).
Naftelberg, S., Schor, I. E., Ast, G. & Kornblihtt, A. R. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu. Rev. Biochem. 84, 165–198 (2015).
Wissink, E. M., Vihervaara, A., Tippens, N. D. & Lis, J. T. Nascent RNA analyses: tracking transcription and its regulation. Nat. Rev. Genet. 165, 535–519 (2019).
Oesterreich, F. C. et al. Splicing of nascent RNA coincides with intron exit from RNA polymerase II. Cell 165, 372–381 (2016).
Herzel, L., Straube, K. & Neugebauer, K. M. Long-read sequencing of nascent RNA reveals coupling among RNA processing events. Genome Res. 28, 1008–1019 (2018).
Khodor, Y. L. et al. Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in Drosophila. Gene Dev. 25, 2502–2512 (2011).
Bentley, D. L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 15, 163–175 (2014).
Hoskins, A. A. et al. Ordered and dynamic assembly of single spliceosomes. Science 331, 1289–1295 (2011).
Wahl, M. C., Will, C. L. & Lührmann, R. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 (2009).
Chen, W. et al. Transcriptome-wide interrogation of the functional intronome by spliceosome profiling. Cell 173, 1031–1044 (2018).
Burke, J. E. et al. Spliceosome profiling visualizes operations of a dynamic RNP at nucleotide resolution. Cell 173, 1014–1030 (2018).
Mayer, A. et al. Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell 161, 541–554 (2015).
Churchman, L. S. & Weissman, J. S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–373 (2011).
Nojima, T. et al. Mammalian NET-seq reveals genome-wide nascent transcription coupled to RNA processing. Cell 161, 526–540 (2015).
Hetzel, J., Duttke, S. H., Benner, C. & Chory, J. Nascent RNA sequencing reveals distinct features in plant transcription. Proc. Natl Acad. Sci. USA 113, 12316–12321 (2016).
Zhu, D. et al. The features and regulation of co-transcriptional splicing in Arabidopsis. Mol. Plant 13, 278–294 (2020).
Li, S. et al. Global co-transcriptional splicing in Arabidopsis and the correlation with splicing regulation in mature RNAs. Mol. Plant 13, 266–277 (2020).
Wu, Z. et al. Quantitative regulation of FLC via coordinated transcriptional initiation and elongation. Proc. Natl Acad. Sci. USA 113, 218–223 (2016).
Zhu, J., Liu, M., Liu, X. & Dong, Z. RNA polymerase II activity revealed by GRO-seq and pNET-seq in Arabidopsis. Nat. Plants 4, 1112–1123 (2018).
Bhatt, D. M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Boutz, P. L., Bhutkar, A. & Sharp, P. A. Detained introns are a novel, widespread class of post-transcriptionally spliced introns. Gene Dev. 29, 63–80 (2015).
Drexler, H. L., Choquet, K. & Churchman, L. S. Splicing kinetics and coordination revealed by direct nascent RNA sequencing through nanopores. Mol. Cell 77, 985–998 (2020).
Kalyna, M. et al. Alternative splicing and nonsense-mediated decay modulate expression of important regulatory genes in Arabidopsis. Nucleic Acids Res. 40, 2454–2469 (2012).
Deng, X. et al. Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper pre-mRNA splicing. Proc. Natl Acad. Sci. USA 107, 19114–19119 (2010).
Deng, X. et al. Recruitment of the NineTeen complex to the activated spliceosome requires AtPRMT5. Proc. Natl Acad. Sci. USA 113, 5447–5452 (2016).
Wang, X. et al. SKIP is a component of the spliceosome linking alternative splicing and the circadian clock in Arabidopsis. Plant Cell 24, 3278–3295 (2012).
Sanchez, S. E. et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature 468, 112–116 (2010).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Gaidatzis, D., Burger, L., Florescu, M. & Stadler, M. B. Analysis of intronic and exonic reads in RNA-seq data characterizes transcriptional and post-transcriptional regulation. Nat. Biotechnol. 33, 722–729 (2015).
James, A. B. et al. Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes. Plant Cell 24, 961–981 (2012).
Gahura, O. et al. Prp45 affects Prp22 partition in spliceosomal complexes and splicing efficiency of non-consensus substrates. J. Cell. Biochem. 106, 139–151 (2009).
Siatecka, M., Reyes, J. L. & Konarska, M. M. Functional interactions of Prp8 with both splice sites at the spliceosomal catalytic center. Gene Dev. 13, 1983–1993 (1999).
Boothby, T. C., Zipper, R. S., van der Weele, C. M. & Wolniak, S. M. Removal of retained introns regulates translation in the rapidly developing gametophyte of Marsilea vestita. Dev. Cell 24, 517–529 (2013).
Naro, C. et al. An orchestrated intron retention program in meiosis controls timely usage of transcripts during germ cell differentiation. Dev. Cell 41, 82–93 (2017).
Mauger, O., Lemoine, F. & Scheiffele, P. Targeted intron retention and excision for rapid gene regulation in response to neuronal activity. Neuron 92, 1266–1278 (2016).
Brody, Y. et al. The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing. PLoS Biol. 9, e1000573 (2011).
Göhring, J., Jacak, J. & Barta, A. Imaging of endogenous messenger RNA splice variants in living cells reveals nuclear retention of transcripts inaccessible to nonsense-mediated decay in Arabidopsis. Plant Cell 26, 754–764 (2014).
Yeom, K. H. & Damianov, A. Methods for extraction of RNA, proteins, or protein complexes from subcellular compartments of eukaryotic cells. Methods Mol. Biol. 1648, 155–167 (2017).
Lamesch, P. et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 40, D1202–D1210 (2012).
Cheng, C. Y. et al. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 89, 789–804 (2017).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Braunschweig, U. et al. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res. 24, 1774–1786 (2014).
Middleton, R. et al. IRFinder: assessing the impact of intron retention on mammalian gene expression. Genome Biol. 18, 51 (2017).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinform. 10, 421 (2009).
Zhu, S. et al. PlantAPAdb: a comprehensive database for alternative polyadenylation sites in plants. Plant Physiol. 182, 228–242 (2020).
Acknowledgements
We thank K.-H. Yeom and Z. Wu for advice on chromatin-bound RNA isolation. The group of J.Z. is supported by the National Key R&D Program of China Grant (2019YFA0903903); an NSFC to J.Z. (grant no. 31871234); the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S172); and the Shenzhen Sci-Tech Fund (KYTDPT20181011104005). J.J. is supported by China Postdoctoral Science Foundation (2018M640787). The group of X.C. is supported by the National Natural Science Foundation of China (grant nos. 31788103 and 91540203, to X.C.); the Chinese Academy of Sciences (Strategic Priority Research Program, XDB27030201 and QYZDY-SSW-SMC022, to X.C.); the Youth Innovation Promotion Association of CAS (grant no. 2018131, to X.D.); and the State Key Laboratory of Plant Genomics.
Author information
Authors and Affiliations
Contributions
J.J., Y.L., D.L., X.J. and X.D. performed the experiments. J.J., Y.L., H.Z., Z.Li, Z.Liu and Y.Z. analysed the data. R.X., X.C. and J.Z. oversaw the study. J.J., Y.L. and J.Z. wrote the manuscript, and all of the authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and the unprocessed western blots for Supplementary Fig. 1.
Supplementary Tables
Supplementary Tables 1–3.
Rights and permissions
About this article
Cite this article
Jia, J., Long, Y., Zhang, H. et al. Post-transcriptional splicing of nascent RNA contributes to widespread intron retention in plants. Nat. Plants 6, 780–788 (2020). https://doi.org/10.1038/s41477-020-0688-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0688-1
This article is cited by
-
A role for heritable transcriptomic variation in maize adaptation to temperate environments
Genome Biology (2023)
-
Remodeling of maternal mRNA through poly(A) tail orchestrates human oocyte-to-embryo transition
Nature Structural & Molecular Biology (2023)
-
Long-read direct RNA sequencing reveals epigenetic regulation of chimeric gene-transposon transcripts in Arabidopsis thaliana
Nature Communications (2023)
-
Plant Intron-Splicing Efficiency Database (PISE): exploring splicing of ∼1,650,000 introns in Arabidopsis, maize, rice, and soybean from ∼57,000 public RNA-seq libraries
Science China Life Sciences (2023)
-
A simple and robust method for isolating and analyzing chromatin-bound RNAs in Arabidopsis
Plant Methods (2022)