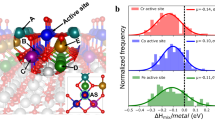
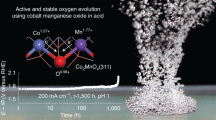
Abstract
Spinel oxides have attracted growing interest over the years for catalysing the oxygen evolution reaction (OER) due to their efficiency and cost-effectiveness, but fundamental understanding of their structure–property relationships remains elusive. Here we demonstrate that the OER activity on spinel oxides is intrinsically dominated by the covalency competition between tetrahedral and octahedral sites. The competition fabricates an asymmetric MT−O−MO backbone where the bond with weaker metal–oxygen covalency determines the exposure of cation sites and therefore the activity. Driven by this finding, a dataset with more than 300 spinel oxides is computed and used to train a machine-learning model for screening the covalency competition in spinel oxides, with a mean absolute error of 0.05 eV. [Mn]T[Al0.5Mn1.5]OO4 is predicted to be a highly active OER catalyst and subsequent experimental results confirm its superior activity. This work sets mechanistic principles of spinel oxides for water oxidation, which may be extendable to other applications.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding authors on reasonable request.
Code availability
The machine-learning codes for making the covalency competition prediction are available at http://github.com/NTUyuanmiao/Covalency_Competition_Dominates_the_Water_Oxidation_Structure-Activity_Relationship_on_Spinel_Oxides.
Change history
04 November 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Armstrong, R. C. et al. The frontiers of energy. Nat. Energy 1, 15020 (2016).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Staffell, I. et al. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12, 463–491 (2019).
Dau, H. et al. The mechanism of water oxidation: from electrolysis via homogeneous to biological catalysis. ChemCatChem 2, 724–761 (2010).
Lee, Y., Suntivich, J., May, K. J., Perry, E. E. & Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 3, 399–404 (2012).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Reier, T., Oezaslan, M. & Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal. 2, 1765–1772 (2012).
Yang, L. et al. Efficient oxygen evolution electrocatalysis in acid by a perovskite with face-sharing IrO6 octahedral dimers. Nat. Commun. 9, 5236 (2018).
Li, H. et al. Metal–oxygen hybridization determined activity in spinel-based oxygen evolution catalysts: a case study of ZnFe2–xCrxO4. Chem. Mater. 30, 6839–6848 (2018).
Zhao, Q., Yan, Z., Chen, C. & Chen, J. Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 117, 10121–10211 (2017).
Chen, J. Y., Miller, J. T., Gerken, J. B. & Stahl, S. S. Inverse spinel NiFeAlO4 as a highly active oxygen evolution electrocatalyst: promotion of activity by a redox-inert metal ion. Energy Environ. Sci. 7, 1382–1386 (2014).
Zhou, Y. et al. Enlarged Co–O covalency in octahedral sites leading to highly efficient spinel oxides for oxygen evolution reaction. Adv. Mater. 30, 1802912 (2018).
Duan, Y. et al. Mastering surface reconstruction of metastable spinel oxides for better water oxidation. Adv. Mater. 31, 1807898 (2019).
Grimaud, A. et al. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 4, 2439 (2013).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Yang, C. & Grimaud, A. Factors controlling the redox activity of oxygen in perovskites: from theory to application for catalytic reactions. Catalysts 7, 149 (2017).
Goodenough, J. B. & Loeb, A. L. Theory of ionic ordering, crystal distortion, and magnetic exchange due to covalent forces in spinels. Phys. Rev. 98, 391–408 (1955).
Rong, X., Parolin, J. & Kolpak, A. M. A fundamental relationship between reaction mechanism and stability in metal oxide catalysts for oxygen evolution. ACS Catal. 6, 1153–1158 (2016).
Zhou, Y. et al. Superexchange effects on oxygen reduction activity of edge‐sharing [CoxMn1−xO6] octahedra in spinel oxide. Adv. Mater. 30, 1705407 (2018).
Wei, C. et al. Cations in octahedral sites: a descriptor for oxygen electrocatalysis on transition‐metal spinels. Adv. Mater. 29, 1606800 (2017).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Lee, Y.-L., Kleis, J., Rossmeisl, J., Shao-Horn, Y. & Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 4, 3966–3970 (2011).
Suntivch, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Suntivch, J., Perry, E. E., Gasteiger, H. A. & Shao-Horn, Y. The influence of the cation on the oxygen reduction and evolution activities of oxide surfaces in alkaline electrolyte. Electrocatalysis 4, 49–55 (2013).
Yang, C., Fontaine, O., Tarascon, J. & Grimaud, A. Chemical recognition of active oxygen species on the surface of oxygen evolution reaction electrocatalysts. Angew. Chem. Int. Ed. 56, 8652–8656 (2017).
Garcia, A. C., Touzalin, T., Nieuwland, C., Perini, N. & Koper, M. Enhancement of oxygen evolution activity of nickel oxyhydroxide by electrolyte alkali cations. Angew. Chem. Int. Ed. 58, 12999–13003 (2019).
Ahneman, D. T., Estrada, J. G., Lin, S., Dreher, S. D. & Doyle, A. G. Predicting reaction performance in C–N cross-coupling using machine learning. Science 360, 186–190 (2018).
Gawande, M. B. et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 116, 3722–3811 (2016).
Sun, S. et al. Shifting oxygen charge towards octahedral metal: a way to promote water oxidation on cobalt spinel oxides. Angew. Chem. 131, 6103–6108 (2019).
Dong, R. et al. Enhanced supercapacitor performance of Mn3O4 nanocrystals by doping transition-metal ions. ACS Appl. Mater. Inter. 5, 9508–9516 (2013).
Liao, H. et al. A multisite strategy for enhancing the hydrogen evolution reaction on a nano‐Pd surface in alkaline media. Adv. Energy Mater. 7, 1701129 (2017).
Laffont, L. & Gibot, P. High resolution electron energy loss spectroscopy of manganese oxides: application to Mn3O4 nanoparticles. Mater. Charact. 61, 1268–1273 (2010).
Wei, C. & Xu, Z. J. The comprehensive understanding of 10 mA cm−2 geo as an evaluation parameter for electrochemical water splitting. Small Methods 2, 1800168 (2018).
Sun, S., Li, H. & Xu, Z. J. Impact of surface area in evaluation of catalyst activity. Joule 2, 1024–1027 (2018).
Jung, S., McCrory, C. C., Ferrer, I. M., Peters, J. C. & Jaramillo, T. F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 4, 3068–3076 (2016).
Wei, C. et al. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 48, 2518–2534 (2019).
Stoerzinger, K. A., Qiao, L., Biegalski, M. D. & Shao-Horn, Y. Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 5, 1636–1641 (2014).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Diaz-Morales, O., Ledezma-Yanez, I., Koper, M. & Calle-Vallejo, F. Guidelines for the rational design of Ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal. 5, 5380–5387 (2015).
Görlin, M. et al. Tracking catalyst redox states and reaction dynamics in Ni–Fe oxyhydroxide oxygen evolution reaction electrocatalysts: the role of catalyst support and electrolyte pH. J. Am. Chem. Soc. 139, 2070–2082 (2017).
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-contaning molecules on transition-metal surfaces. Phys. Rev. Lett. 99, 016105 (2007).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–armorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S., Botton, G., Savrasov, S., Humphreys, C. & Sutton, A. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223–16233 (1994).
Anaconda Software Distribution. Computer software. v.2-2.4.0. (Anaconda, 2016); https://anaconda.com
Svetnik, V. et al. Random forest: a classification and regression tool for compound classification and QSAR modeling. J. Chem. Inf. Comp. Sci. 43, 1947–1958 (2003).
Jha, D. et al. Elemnet: deep learning the chemistry of materials from only elemental composition. Sci. Rep. 8, 17593 (2018).
Islam, M. et al. Study on the electrochemical reaction mechanism of NiFe2O4 as a high-performance anode for Li-ion batteries. ACS Appl. Mater. Inter. 9, 14833–14843 (2017).
Du, Y. et al. XAFCA: a new XAFS beamline for catalysis research. J. Synchrotron Rad. 22, 839–843 (2015).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Yu, X., Diao, C., Venkatesan, T., Breese, M. & Rusydi, A. A soft X-ray-ultraviolet (SUV) beamline and diffractometer for resonant elastic scattering and ultraviolet-vacuum ultraviolet reflectance at the Singapore synchrotron light source. Rev. Sci. Instrum. 89, 113113 (2018).
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 31, 1806296 (2019).
Mishra, R. K. & Thomas, G. Surface energy of spinel. J. Appl. Phys. 48, 4576–4580 (1977).
Farragher, A. Surface vacancies in close packed crystal structures. Adv. Colloid Interface Sci. 11, 3–41 (1979).
Roy, C. et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 1, 820 (2018).
Friebel, D. et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Acknowledgements
This work was supported by Singapore Ministry of Education Tier 2 Grant (MOE-2018-T2-2-027) and the Singapore National Research Foundation under its Campus for Research Excellence And Technological Enterprise (CREATE) programme. We thank the Facility for Analysis, Characterization, Testing, and Simulation (FACTS) in Nanyang Technological University. This research used resources of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. We also appreciate the XAS measurements from SSLS, soft X-ray and ultraviolet beamline. Y.S. and Z.X. thank A. Lapkin (University of Cambridge) for helpful discussion on machine-learning concepts and thank L. Zeng (Southern University of Science and Technology) for helpful discussion on catalyst performance. H.Z. gives thanks for the support from ITC via the Hong Kong Branch of National Precious Metals Material (NPMM) Engineering Research Center, and the start-up grant (project no. 9380100) and grants (project no. 9610478 and 1886921) in City University of Hong Kong.
Author information
Authors and Affiliations
Contributions
Z.J.X. and Y.S. proposed the research. Y.S., H.L. and Z.J.X. designed the experiments. Y.S. conducted DFT modelling and simulations. H.L. established the mathematical approach. H.L., J.W., S.S., B.C. and S.J.H.O. carried out the experiments. S.X., C.D., Y.D., J.W., J.O.W., Y.S. and H.L. conducted XAS characterizations. Y.S. wrote the manuscript. H.L., S.X., Y.D., M.B.H.B., S.L., H.Z. and Z.J.X. performed the analysis and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–11 and Figs. 1–12.
Supplementary Data 1
Atomic coordinates of the calculated bulk spinels.
Supplementary Data 2
Atomic coordinates of the OER intermediates.
Rights and permissions
About this article
Cite this article
Sun, Y., Liao, H., Wang, J. et al. Covalency competition dominates the water oxidation structure–activity relationship on spinel oxides. Nat Catal 3, 554–563 (2020). https://doi.org/10.1038/s41929-020-0465-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0465-6
This article is cited by
-
Stabilizing high-efficiency iridium single atoms via lattice confinement for acidic oxygen evolution
Nano Research (2024)
-
Unraveling the role of NiSnPH@OOH/CC perovskite hydroxide for efficient electrocatalytic oxidation of methanol to formate
Nano Research (2024)
-
Bridging the complexity gap in computational heterogeneous catalysis with machine learning
Nature Catalysis (2023)
-
Navigating surface reconstruction of spinel oxides for electrochemical water oxidation
Nature Communications (2023)
-
High-entropy single-atom activated carbon catalysts for sustainable oxygen electrocatalysis
Nature Sustainability (2023)